Figure 3.
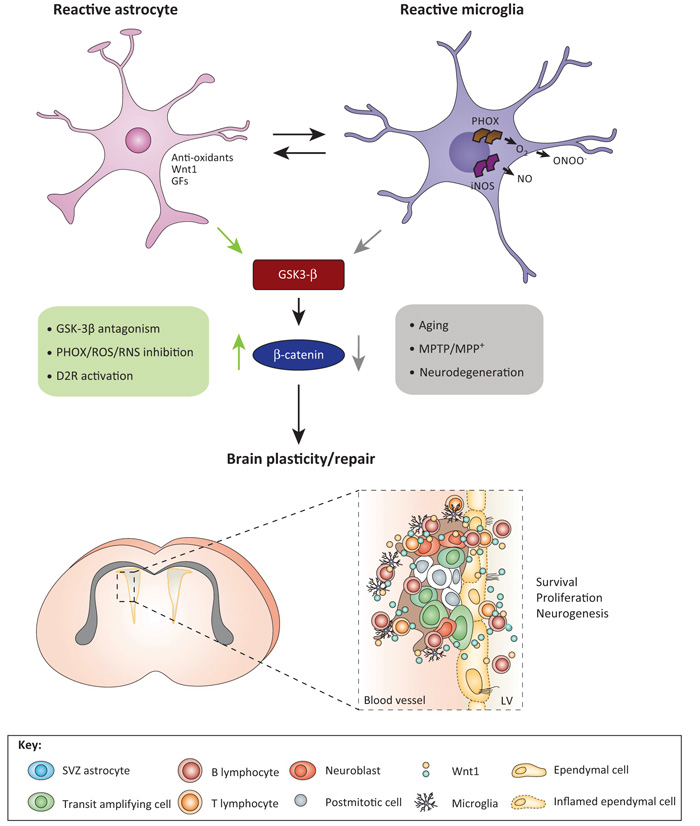
Crosstalk between Wnt and local inflammatory signaling pathways for the control of SVZ niche homeostasis. During the early degeneration phase of MPTP toxicity, hyperactivated microglia contribute to the impairment of SVZ neurogenesis at different levels. By increasing oxidative and nitrosative stress and in synergy with MPTP/MPP+ direct toxicity, microglial-derived mediators (PHOX-derived ROS and iNOS-derived NO and peroxynitrite) may act as a molecular switch for cell signaling pathways critically involved in the physiological control of NPC homeostasis, with harmful consequences for NPC physiology, at least in part through GSK-3β activation, followed by phosphorylation and consequent degradation of β-catenin [10,15]. By contrast, pharmacological mitigation of inflammation and oxidative stress with Apo, L-Nil, or HCT1026 upregulate β-catenin and successfully rescue NPC proliferation and neuroblast formation, a process associated with striatal DAergic neuroprotection, with further positive modulation of SVZ proliferation via D2-R-activated mechanisms [10]. The mutual role of astrocyte–microglial interactions in the plasticity of SVZ response to MPTP is exemplified by the astrocyte’s ability to overcome microglial inhibitory effects, through, besides others, Wnt1-mediated activation Wnt/β-catenin signaling [10]. Different upstream and downstream signaling cascades may converge in finely tuning β-catenin transcriptional activity. For example, NO can inhibit EGF-R and the phosphoinositide 3-kinases (PI3K)/AKT survival pathway, and GSK-3β is a downstream target of Akt; RNS-induced nitration regulates the p85 subunit of PI3 kinase; and a variety of molecules including growth factors and neurotransmitter, including DA via D2 receptor can signal through (PI3K)/AKT/GSK-3β and Wnt signaling activation, thus, crosstalk among Wnt/β-catenin and prominent intracellular pathways may be envisioned to fine tune the SVZ neurogenic potential/plasticity observed herein [10]. Crosstalk between SVZ astrocytes (blue), transit-amplifying cells (red), neuroblasts (orange), ependymal (yellow) cells, microglia (violet), and lymphocytes via Wnt in SVZ niche are schematically illustrated. Abbreviations: Apo, apocynin, a ROS antagonist; DA, dopamine; D2-R, dopaminergic receptor subtype 2; HCT1026, [2-fluoro-α-methyl(1,1′-biphenyl)-4-acetic-4-(nitrooxy)butyl ester], a mixed cyclooxygenase (COX1/COX2) inhibitor NO-donating non-steroidal anti-inflammatory drug (NSAID) endowed with additional anti-inflammatory activity and reduced side effects; L-Nil, L-N6-(1-iminoethyl)-lysine, a specific inhibitor of inducible nitric oxide synthase (iNOS); MPP+, 1-methyl-4-phenylpyridinium; MPTP, 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine; NPC, neural stem/progenitor cell; PHOX, phagocyte oxidase; RNS, reactive nitrogen species; ROS, reactive oxygen species; SVZ, subventricular zone. Adapted from [111].