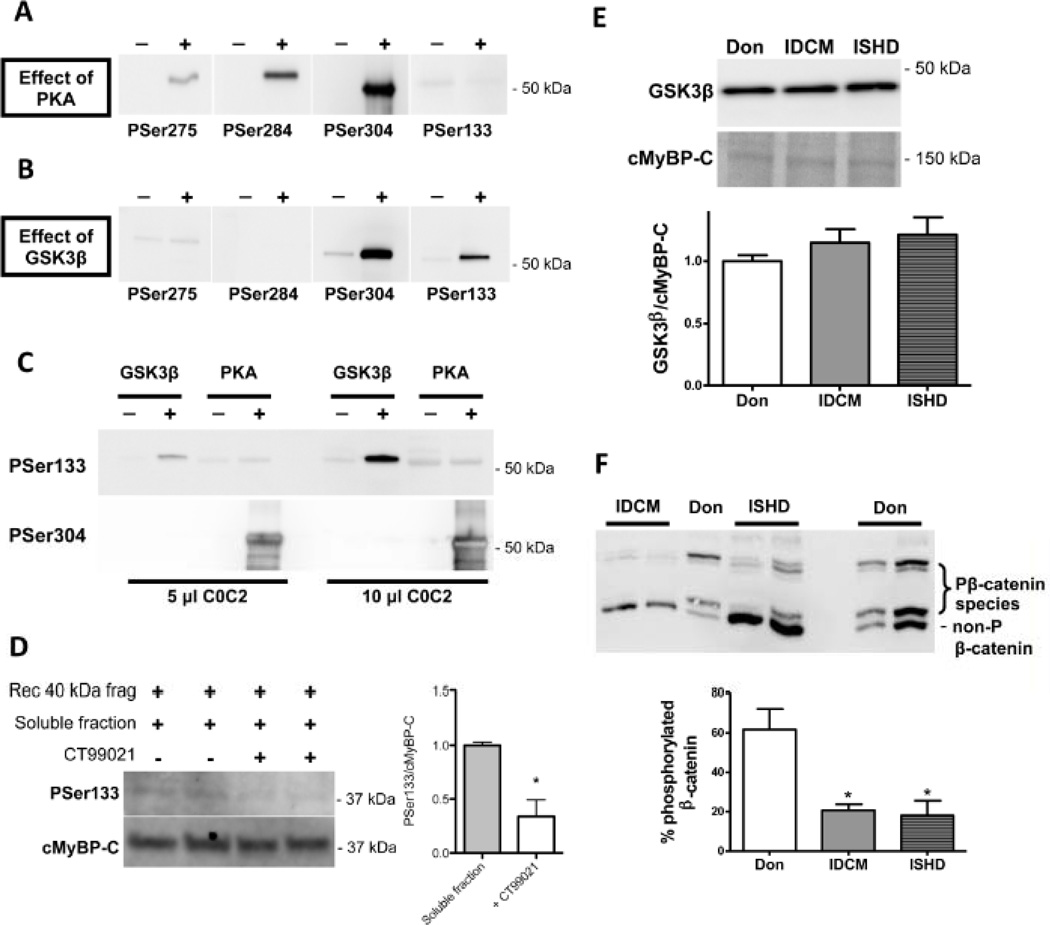
Figure 3. Ser133 is phosphorylated by GSK3β.
Human recombinant C0C2 fragment was incubated with either PKA or GSK3β for 2 hours at 37°C. Phosphorylation at serines 275, 284, 304 and 133 was determined with phospho-specific antibodies. A. PKA phosphorylated Ser275, 284 and 304, but not Ser133. B. GSK3β phosphorylated Ser304 and 133. C. To directly compare the relative capability of PKA and GSK3β to phosphorylate Ser133 and Ser304, human recombinant C0C2 was incubated without kinase, with PKA (10 pmol/min/µg) or with GSK3β (168 pmol/min/µg) and loaded onto the same gel in two different amounts followed by immunoblotting with site-specific antibodies. Ser133 was only phosphorylated by GSK3β while Ser304 was predominately phosphorylated by PKA. D. Recombinant 40kDa cMyBP-C (amino acids 1–271 from the human sequence) was incubated with a rough cytosolic fraction isolated from donor heart tissue with (n=3) or without (n=3) 2µM CT99021 (GSK3β antagonist) to determine whether endogenous GSK3β is responsible for Ser133 phosphorylation. PSer133 signal was normalized to total cMyBP-C (with antibody against C0 domain) and 40kDa + soluble fraction was set at 1. CT99021 significantly decreased PSer133 phosphorylation. *P<0.05, with versus without CT99021 in unpaired Student’s t-test. E. GSK3β protein levels were not changed in the end-stage failing hearts compared with donor hearts. F. Phosphorylation of another GSK3β target protein β-catenin was studied by Phos-tag analysis. Reversible interaction between phosphorylated proteins and Phos-tag reagent slow down migration of phosphorylated proteins through the gel. End-stage failing hearts showed a greater proportion of unphosphorylated β-catenin compared with donor hearts suggesting a reduced GSK3β activity. *P<0.01 IDCM and ISHD versus donor in 1-way ANOVA followed by Bonferroni post-hoc test.