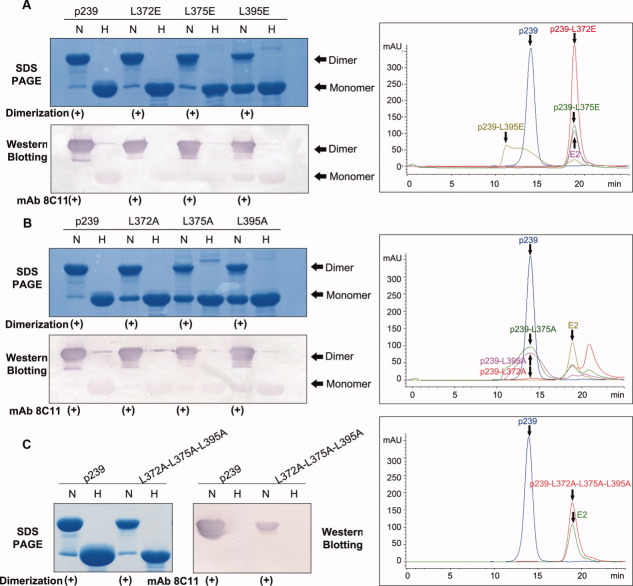
Figure 6.
Mutational studies of the N-terminal region of p239. (A) SDS-PAGE, Western blotting, and GFC analysis of three single-site Glu mutants (p239-L372E, p239-L375E, and p239-L395E). (B) SDS-PAGE, Western blotting and GFC analysis of three single-site Ala mutants (p239-L372A, p239-L375A, and p239-L395A). (C) SDS-PAGE, Western blotting, and GFC analysis of the triple-site Ala mutant (p239-L372A-L375A-L395A). The lanes labeled with H indicate samples in the reduced condition that were heated to 100°C for 3 min, and these samples were primarily resolved as monomers. The lanes labeled with N indicate samples under non-reducing conditions, which only contained 0.1% SDS, and the samples were not heated. p239 and its mutants were reacted with the neutralizing mAb 8C11 of HEV. Western blotting analysis showed that all the mutants remained the strong reactivity with mAb 8C11 with respect to the prototype p239. Three single-site Ala mutants could partially assemble into particles with similar elution time to p239 particles, while three single-site Glu mutants and the triple-site Ala mutant failed to form p239-like particles. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]