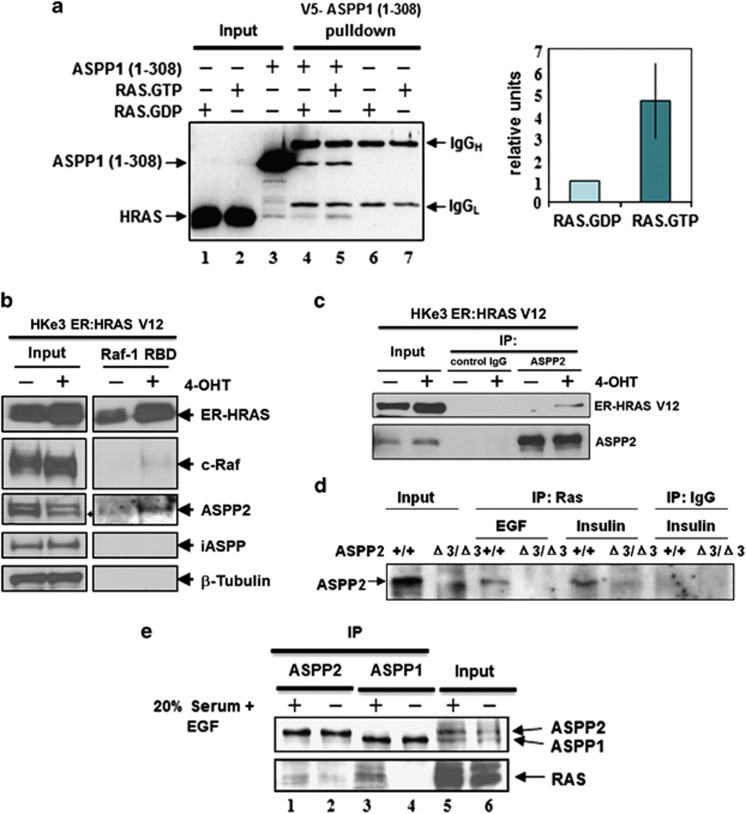
Figure 1.
ASPP1 and ASPP2 preferentially bind active RAS. (a) ASPP1's N-terminus preferentially binds RAS–GTP in vitro. RAS protein, loaded with either tritium-labelled GDP or GTP, was added to V5-tagged recombinant ASPP1 (1–310) and immunoprecipitated with V5 antibody. As a negative control, RAS.GDP and RAS–GTP were immunoprecipitated with V5 antibody in the absence of ASPP1 (1–310). Co-immunoprecipitated RAS was quantified according to the presence of 3H-GTP or 3H-GDP. Values are shown as a bar graph. Standard deviation represents the mean of three independent experiments. (b) ASPP2 binds activated RAS in HKe3 cells. HKe3 ER:HRASV12 cells were treated with 100 nM 4-OHT for 2 days and Raf-1–RBD agarose pull-down assays were performed to pull down GTP-bound active RAS. (c) ASPP2 binds activated ER:HRASV12. Total cell lysates from HKe3 ER:HRASV12 cells treated with or without 4-OHT were immunoprecipitated with an anti-ASPP2 antibody, or control IgG as indicated. (d) ASPP2 binds activated RAS in MEFs. Total cell lysates from ASPP2(+/+) or ASPP2(Δ3/Δ3) MEFs treated with EGF (20 ng/ml) or insulin (1 μg/ml) for 15 min were immunoprecipitated with an anti-HRAS antibody or control IgG. (e) Saos2 cells were either starved in 0.5%, or stimulated with 20%, FCS plus 20 ng/ml EGF overnight. Lysates were collected and immunoprecipitated with rabbit anti-ASPP1 (ASPP1.88) or anti-ASPP2 (ASPP2/77) antibodies, respectively. The ASPP proteins were detected with mAbASPP1.54.1, which is known to crossreact with both ASPP1 and ASPP2