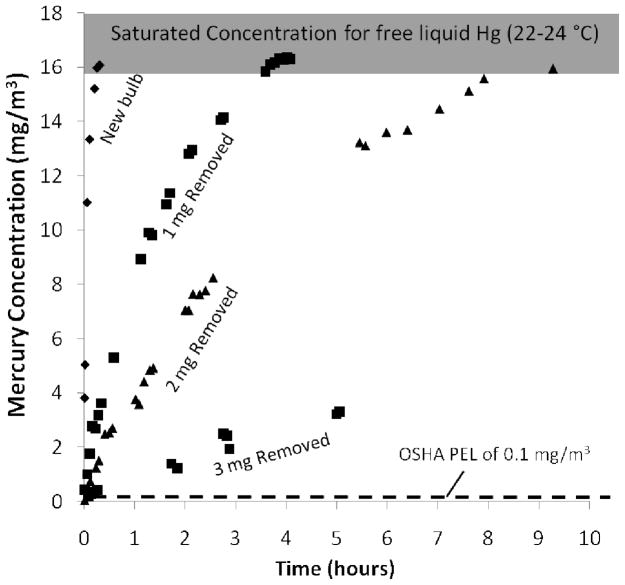
Figure 2.
Equilibrium mercury vapor concentrations above CFL debris, and rate of approach to the equilibrium values in a 0.5 L flask. Although most Hg is associated with the phosphor, glass, or endcaps, its equilibrium vapor pressure is similar to that of free liquid Hg and over 150 times the OSHA-PEL. Partial removal of Hg (1, 2, and 3mg) by heating and gas purging reduces the release rates, but does not affect the equilibrium vapor concentrations. After 3 mg removal, the equilibrium Hg concentration returns to >15,000, but over 30 hours (data not shown). This equilibrium data is relevant to Hg-vapor concentrations expected in small confined spaces.