Abstract
Background:
The objective of this study was to assess the magnitude of the cutaneous leishmaniasis (CL) disease and identification of the causative agent by nested-PCR for current control strategy.
Methods:
This study was carried out as descriptive house-to-house visits in Orzoieh district in Kerman Province, south–east Iran, during 2011–2012. A questionnaire was completed for each individual consisting of demographic and clinical data. Suspected individuals were examined by direct smear microscopy and subsequent identification by nested-PCR. X2-test was used for any significance (P<0.05).
Results:
A total of 18308 inhabitants (mean age; 22.7 yr) consisting of 9011 males (49.2%) and 9297 females (50.8%) were examined for the presence of active or chronic lesions. The overall prevalence was 4.7%, including 30 cases of active and 839 cases of scar, distributed more significantly (P<0.01) in females (5.2%) than males (4.3%). Individuals <10 years of age showed the highest (6.3%) and >50 years the lowest rate of CL disease, respectively (P<0.001). The proportion of infection was the highest in Soltanabad (14.7%), followed by Vakilabad (6.8%), Dolatabad (3.2%) and Shahmaran (2.8%). The majority of cases had 2 lesions (mean; 2.1 lesions). Hand was the most common site of involvement (35%), and then face (26%), and multiple locations (39%). Nested-PCR displayed 29 isolates as Leishmania major and one isolate L. tropica. The CL disease first emerged in 1998 as epidemic in the area and appeared endemics, thereafter.
Conclusion:
L. major was the sole species caused ZCL. These findings are necessary for future control programs and strategic planning.
Keywords: Cutaneous leishmaniasis, Leishmania major, Epidemiology, Iran
Introduction
Cutaneous leishmaniasis (CL) caused by Leishmania species represents a complex disease with wide spectrum of clinical features (1). It is endemic in over 88 countries, mainly in tropical and sub-tropical areas (2), where over 90% of the cases occur in Afghanistan, Brazil, Algeria, Peru, Iran, Saudi Arabia and Syria (3). Surveillance data indicate the number of cases has sharply increased in recent years, as documented in several countries (1).
Cutaneous leishmaniasis is endemic in over 50% of the provinces of Iran (4, 5); anthroponotic CL (ACL) caused by L. tropica, exists mainly in urban areas of many large and medium size cities (6–9). The main host is human (2, 5, 10). Whereas, zoonotic CL (ZCL) due to L. major presents in rural areas (11) where the major reservoir host is gerbil (12–14). Several factors which contribute to the spread of CL throughout the world are urbanization, migration, war situation, environmental modification and also to some extent improved diagnosis and case notification (15). Outbreaks of CL have currently occurred throughout Iran, where a suitable environment is present for breeding of the vector, propagation of the organism and transmission of the causative agent (16–18).
The primary objective of this study was to assess the present status of CL in endemic areas of Baft, currently Orzoieh district. First emergence was started as epidemic in 1998 and the disease became endemic, thereafter (17). After the massive earthquake, it seemed that the feature of CL has been changing in this area, mainly because of the recent expansion of villages and towns, as well as movement of the population for various occupational reasons. Due to the spread of the CL cases to the neighboring regions and reports by the physicians and health authorities in the area, the CL lesions present different clinical and epidemiological characteristics.
Therefore, the aim of the present study was to assess the magnitude of the CL infection and to identify the causative agent by a molecular method for future control strategy and current therapeutic measures.
Materials and Methods
Study areas
This study was carried out in Orzoieh district, in south-west Kerman Province, south–east of Iran. This district is recently segregated from Baft and consists of 4 main regions including, Vakilabad, Soltanabad, Dolatabad and Shahmaran. Orzoieh surrounds 4600 km2 area, located 125 km south to the city of Baft, adjacent to Jiroft district and Hormozgan Province. The climate is warm in the summer, while mild in the winter. Due to the productivity of soil in this region, 6000 hectares of the land is cultivated by all kinds of tropical crops including orchard of oranges, summer crops and various vegetables. Its irrigation system is provided by 700 water wells. This region has recently been attracted by residents of Isfahan, Yazd and people from other provinces for agricultural purposes. Thus, numerous labors moved to the region for occupational reasons.
Population and sampling
This study was carried out as descriptive and prospective census in house- to – house visits during March 2011 to February 2012 for a period one year. The entire population of the affected villages was screened and followed-up actively and passively. A questionnaire was completed for each individual, recording demographic characteristics (sex, age, location and residence) and clinical status of CL (location of lesions, number and type of lesions and time of contraction). The entire body was clinically examined by two health personnel and those with suspected lesions were referred for further clinical examination by an experienced physician and parasitological examination.
Smear preparation
Suspected lesions were subjects for smear preparation. Skin scrapings were taken by a scalpel and blade from the edge of each active lesion, smeared on a glass slide, fixed with methanol and stained by Giemsa.
Molecular identification
Direct skin smears obtained from the borders of lesions were used for nested-PCR. All smears were transferred o the Leishmaniasis Research Center at Medical School in Kerman for further molecular study. DNA was extracted by proteinase K (Roche, Germany) from the smear preparations following the method of Noyes et al (19). Briefly, two types of primers were used in two sequential steps. First, two external general primers; CSB2XF 5′-CGAGCAGCAGAAACTCCCGTTCA-3′ and CSB1XR 5-′ATTTTTCGCGATTTTCGCAGAACG-3′ and second, two other internal specific primers; 13Z 5′-ACTGGGGGTTGGTGTAAAATAG-3′ and LiR5′TCGCAGAACGCCCCT-3′ were used. These primers amplified a variable minicircle k DNA of Leishmania species.
The PCR products were visualized by 1.5% agarose gel electrophoresis (Uvitech, Cambridge, UK), using a DNA size marker ( 100bp) at 260nm wavelength.
Ethical issues
The protocol was reviewed and subsequently approved by the Ethic Committee of Kerman University of Medical Sciences. The consent of patients was obtained and the confirmed cases were referred to a local physician for treatment of CL.
Data analysis
A SPSS software was used for data entry and X2-test was performed for any significant differences at P<0.05.
Results
A total of 18308 inhabitants, aged 1–73 years (mean age; 22.7 years) including of 9011 males (49.2%) and 9297 females (50.8%) were examined for presence of active or chronic lesions (Table 1). Most of the individuals were < 30 years (64.2%) and the smallest age group was >50 years (13.2%). The overall prevalence rate was 4.7% including 30 cases of active lesions and 839 cases of scar, distributed among males (4.3%) and females (5.2%) with a significant difference (P< 0.01, Table 2).
Table 1:
Age and sex distribution of the study population in Orzoieh district, Kerman province, south-eastern Iran, 2011–2012
| Age groups (yr) | Male | Female | Total | |||
|---|---|---|---|---|---|---|
| n | % | n | % | n | % | |
| ≤10 | 1591 | 17.6 | 2004 | 21.6 | 3595 | 19.6 |
| 11–20 | 1917 | 21.3 | 1724 | 18.5 | 3641 | 19.9 |
| 21–30 | 2264 | 25.1 | 2258 | 24.3 | 4522 | 24.7 |
| 31–40 | 1240 | 13.8 | 1281 | 13.8 | 2521 | 13.8 |
| 41–50 | 809 | 9.0 | 804 | 8.6 | 1613 | 8.8 |
| >50 | 1190 | 13.2 | 1226 | 13.2 | 2416 | 13.2 |
| Total | 9011 | 100.0 | 9297 | 100.0 | 18308 | 100.0 |
Table 2:
Distribution of cutaneous leishmaniasis in Orzoieh district, Kerman Province, south-eastern Iran by sex, 2011–2012
| Gender | No. examined | No. infected (%) |
|---|---|---|
| Male | 9011 | 384(4.3) |
| Female | 9297 | 485(5.2) |
| Total | 18308 | 869(4.7) |
All age groups were infected (Table 3), however subjects <10 years of age indicated the highest (6.3 %) and >50 years the lowest rate of CL infection (1.1%), respectively. Overall, there was a significant difference between <10 years old children and other age groups (P< 0.001).
Table 3:
Distribution of cutaneous leishmaniasis in Orzoieh district, Kerman Province, south-eastern Iran by age, 2011–2012
| Age groups (yr) | No. examined | No. infected (%) |
|---|---|---|
| ≤10 | 3595 | 228(6.3) |
| 11–20 | 3641 | 209(5.7) |
| 21–30 | 4522 | 206(4.6) |
| 31–40 | 2521 | 112(4.4) |
| 41–50 | 1631 | 88(5.5) |
| >50 | 2416 | 26(1.1) |
| Total | 18308 | 869(4.7) |
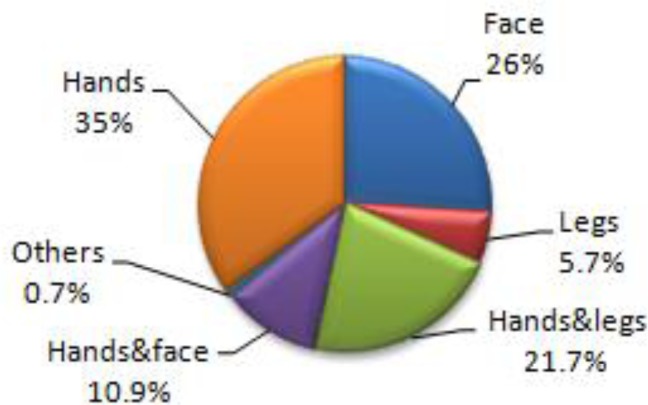
The proportion of infection was the highest in Soltanabad (14.7%), followed by Vakilabad (6.8%), Dolatabad (3.2%) and Shahmaran (2.8%). The majority of cases (38.2%) had 2 lesions (mean; 2.1). The hands (35%) were the most common place of involvement, followed by face(26%), hands and legs ( 21.7%), hands and face (10.9%), legs (5.7%) and other parts of the body (0.7%, Fig. 1).
Fig. 1:
Distribution of cutaneous leishmaniasis in Orzoieh distract, Kerman Province, south-eastern Iran by location of lesion, 2011–2012
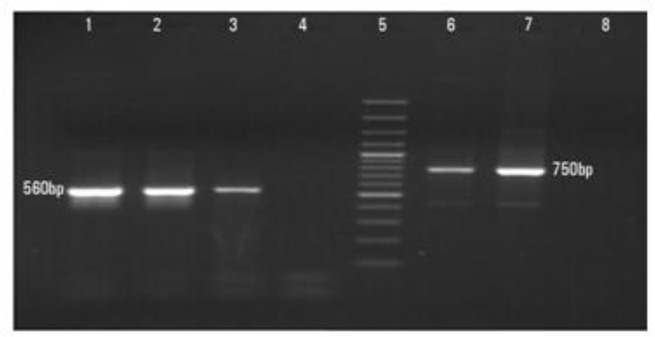
DNA extracted from direct smear preparations showed 29 cases consisting of fragments of 560 bp, while only one case displayed a fragment of 750 bp, corresponding to L. major (99.9%) and L. tropica (0.1%), respectively (Fig. 2).
Fig. 2:
Electrophoresis gel fractionation of nested-PCR for identification of Leishmania isolates. Lane1, L. major (positive control 560 bp); Lanes 2, 3, L. major isolates; Lanes 4,8, distilled water (negative control); Lane 5 DNA size marker 100bp: Lane 6 L. tropica (positive control 750 bp) ; Lane 7 single L. tropica isolate obtained from a patient in Orzoieh district, Kerman Province, south-eastern Iran, 2011–2012
Infected individuals were entirely natives. The CL cases first appeared during 1998, but the majority occurred thereafter.
Discussion
Leishmaniasis is still a significant cause of morbidity and mortality in numerous countries across the world, including Iran (2, 3). CL also affects a considerable portion of human beings with a major health impact in recent years (1).
Although, L. tropica is the main species in endemic foci of various districts in Kerman Province (8), the district of Orzoieh is determined to be purely of ZCL nature. The only case detected as L. tropica was a native resident who had history of travelling to Jiroft, an area adjacent to this district with a known ACL focus (20).
The CL cases were commonly found in females more significantly than males. However, there is no definite explanation for such a sex difference. It might be attributed to individual and behavioral risk factors which could play a role as previously reported by others (16, 21).
All age groups were infected, although there was a strong tendency for cases to be prevalent more significantly in children of <10 years than other age groups. This is likely expected because children in this age play outdoors and are more exposed to the source of infection than others. Similar findings are found by others (11,12,17,21).
The clinical manifestations of CL were in general that of multiple lesions, with a predominant location on hands, face, hands-legs or hands-face (11, 12). These extrinsic factors are consistent with the molecular identification by nested – PCR which showed that L. major was the only causative agent in the area as previously reported in other places (5, 8, 11, 12). In this study nested PCR with high specificity and sensitivity was applied to identify the causative parasite species among the inhabitants. This method is currently the most commonly used technique in discrimination of species (8, 11, 13, 18, 19).
The present status of the CL cases indicates that the study area is designated a recently emerged focus occurred for the first time in 1998 (17) and sustained as endemic, thereafter (12–13). It appeared that the source of infection was introduced by the new settlements that arrived in the area from endemic regions for agricultural purposes (17). In addition, the environmental and ecological changes favored an increase in the vector population and reservoir host, gerbils (12–13) which might also have played some roles in propagation of the life cycle and transmission of the causative organisms.
The semi nested-PCR was positive for 0.05% of Phlebotomus papatasi in the region, a further evidence for a ZCL focus (13). Furthermore, another investigation indicated that P. papatasi was the predominant species and they reported prevalence of 1.1% for ulcers and 10.4% for scars among inhabitants in 2003 (12). The same study indicated that Tatera indica was the common gerbil in this area. They concluded that this gerbil might have played a role as the reservoir host in the focus.
Conclusion
L. major was the principle species caused ZCL in the study areas. This kind of information clearly has therapeutic significance and helpful in selection of optimal therapy. These findings are also necessary for designing future control programs and strategic planning.
Ethical considerations
Ethical issues (Including plagiarism, Informed Consent, misconduct, data fabrication and/or falsification, double publication and/or submission, redundancy, etc) have been completely observed by the authors.
Acknowledgments
This study was supported by Kerman University of Medical Sciences (project no 89/254). We thank the health personnel in Orzoieh Health System for their kind assistance. The authors declare that there is no conflict of interest.
References
- 1.Reithinger R, Dujardin JC, Louzir H, Primez C, Alexander B, Brooker S. Cutaneous leishmaniasis. Lancet Infect Dis. 2007;7(9):581–96. doi: 10.1016/S1473-3099(07)70209-8. [DOI] [PubMed] [Google Scholar]
- 2.World Health Organization (WHO) Control of the leishmaniases. 2010. pp. 1–187. Report of a Meeting of the WHO Expert Committee on the Control of Leishmaniasis, WHO Technical Report Series 949, Geneva.
- 3.Desjeux P. Leishmaniasis: current situation and new perspectives. Comp Immunol Microbiol Infect Dis. 2004;27:305–318. doi: 10.1016/j.cimid.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 4.Motazedian H, Noamanpoor B, Ardehali S. Characterization of Leishmania parasites isolated from Provinces of the Islamic Republic of Iran. East Mediterr Health J. 2002;8(2–3):338–44. [PubMed] [Google Scholar]
- 5.Shirzadi MR, Gouya MM. National Guidelines for cutaneous leishmaniasis surveillance in Iran, MOHAME. Zoonoses Control Department; Tehran, I.R. Iran: 2010. pp. 1–59. [Google Scholar]
- 6.Hajjaran H, Mohebali M, Razavi MR, Rezaei S, Kazemi B, Edrissian GH, Mojtabavi J, Hooshmand B. Identification of Leishmania species isolated from human cutaneous leishmaniasis, using random amplified polymorphic DNA (RAPD-PCR) Iranian J Publ Health. 2004;33(4):8–15. [Google Scholar]
- 7.Nadim A, Aflatoonian MR. Anthroponotic cutaneous leishmaniasis in Bam, southeast Iran. Iranian J Publ Health. 1995;24:15–24. [Google Scholar]
- 8.Sharifi F, Sharifi I, Zarean M, Hakimi Parizi M, Aflatoonian MR, Fasihi Harandi M, Zahmatkesh R, Mashayekhi M, Kermanizadeh AR. Spatial distribution and molecular identification of Leishmania species from endemic foci of south-eastern Iran. Iranian J Parasitol. 2012;7(1):45–52. [PMC free article] [PubMed] [Google Scholar]
- 9.Zahraei-Ramazani AR, Yaghoobi-Ershadi MR, Mokhtari AR, Akhavan AA, Abdoli H, Arandian MH. Anthroponotic Cutaneous Leishmaniasis in Nonendemic Quarters of a Centeral City in Iran. Iranian J Publ Health. 2007;36(2):7–11. [Google Scholar]
- 10.Aghaei Afshar A, Rassi Y, Sharifi I, Abai MR, Oshaghi MA, Yaghoobi-Ershadi MR, Vatandoost H. Susceptibility status of Phlebotomus papatasi and P. sergenti (Diptera: Psychodidae) to DDT and Deltamethrin in a focus of cutaneous leishmaniasis after earthquake strike in Bam, Iran. Iran J Arthropod-Borne Dis. 2011;5(2):32–41. [PMC free article] [PubMed] [Google Scholar]
- 11.Maraghi S, Samarbaf Zadeh A, Sarlak AA, Ghasemian M, Vazirianzadeh B. Identification of cutaneous leishmaniasis agents by nested polymerase chain reaction (Nested-PCR) in Shush city, Khuzestan Province, Iran. Iranian J Parasitol. 2007;2(3):13–15. [Google Scholar]
- 12.Akhavan AA, Yaghoobi-Ershadi MR, Hasibi F, Jafari R, Abdoli H, Arandian MH, Soleimani H, Zahraei-Ramazani AR, Mohebali M, Hajjaran H. Emergence of cutaneous leishmaniasis due to Leishmania major in a new focus of southern Iran. Iranian J Arthropod-Borne Dis. 2007;1(1):1–8. [Google Scholar]
- 13.Oshaghi MA, Yaghobi-Ershadi MR, Abbassi M, Parvizi P, Akhavan AA, Rahimi Foroshani A, Zahraei AR, Rassi Y, Mohtarami F. Detection of Leishmania major In naturally infected sand flies using semi nested-PCR. Iranian J Publ Health. 2008;37(4):59–64. [Google Scholar]
- 14.Yaghoobi-Ershadi MR, Akhavan AA, Abai MR, Zahraei-Ramazani AV, Ebrahimi B, Vafaei Nezhad R, Hanafi-Bojd AA, Jafari R. Epidemiological study in a new focus of cutaneous leishmaniasis in the Islamic Republic of Iran. East Mediterr Health J. 2004;10(4–5):688. [PubMed] [Google Scholar]
- 15.Desjeux P. The increase in risk factors for leishmaniasis worldwide. Trans Roy Soc Trop Med Hyg. 2001;95(3):239–43. doi: 10.1016/s0035-9203(01)90223-8. [DOI] [PubMed] [Google Scholar]
- 16.Fazaeli A, Fouladi B, Sharifi I. Emergence of cutaneous leishmaniasis in a border area at south-east of Iran: an epidemiological survey. J Vector Borne Dis. 2009;46(1):36–42. [PubMed] [Google Scholar]
- 17.Sharifi I, Zemani F, Aflatoonian MR, Fekri AR. An epidemic of cutaneous leishmaniasis in Baft district in Kerman Province and its probable causative risk factors. Iranian J Epidemiol. 2008;4(1):53–58. [Google Scholar]
- 18.Sharifi I, Poursmaelian S, Aflatoonian MR, Ardakani RF, Mirzaei M, Fekri AR, Khamesipour A, Hakimi Parizi M, Harandi MF. Emergence of a new focus of anthroponotic cutaneous leishmaniasis due to Leishmania tropica in rural communities of Bam district after the earthquake, Iran. Trop Med Int Health. 2011;16(4):510–513. doi: 10.1111/j.1365-3156.2011.02729.x. [DOI] [PubMed] [Google Scholar]
- 19.Noyes HA, Reyburn H, Bailey JW, Smith D. A nested-PCRbased schizodeme method for identifying of Leishmania kinetoplast minicircle classes directly from clinical samples and its application to the study of the epidemiology of Leishmania tropica in Pakistan. J Clin Microbiol. 1998;36(10):2877. doi: 10.1128/jcm.36.10.2877-2881.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mirzaei M, Sharifi I, Poursmaelian S. A new focus of anthroponotic cutaneous leishmaniasis and identification of parasite species by nested PCR in Jiroft, Iran. Comp Clin Pathol. 2012;21:1071–1075. [Google Scholar]
- 21.Sharifi I, Nakhaei N, Aflatoonian MR, Hakimi Parizi M, Fekri AR, Safizadeh H, Shirzadi MR, Gooya MM, Khamesipour A, Nadim A. Cutaneous leishmaniasis in Bam: A comparative evaluation of pre and post-earthquake years 1999–2008. Iranian J Publ Health. 2011;40(2):49–56. [PMC free article] [PubMed] [Google Scholar]