Fig. 3.
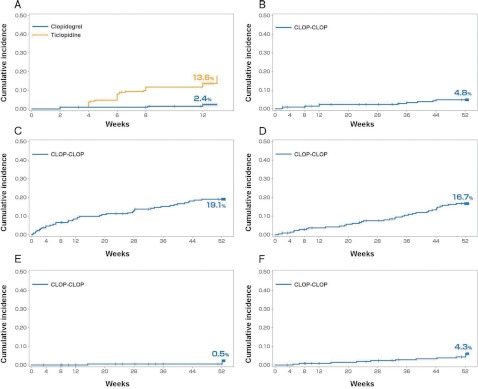
Kaplan-Meier curves of first events up to 12 weeks and 52 weeks: all randomized population.
A: The cumulative incidence of “safety events of interest” in the clopidogrel group was significantly lower than that of the ticlopidine group (2.4% [95% CI, 0.3% to 4.4%] vs. 13.6% [95% CI, 9.0% to 18.2%] at 12 weeks; adjusted HR, 0.161; 95% CI, 0.062 to 0.416; p <0.0001). B: The cumulative incidence of “safety events of interest” for the CLOP-CLOP group up to 52 weeks (4.8% [95% CI, 1.9% to 7.8%]). C: The cumulative incidence of bleeding adverse events for the CLOP-CLOP group up to 52 weeks (19.1% [95% CI, 13.8% to 24.4%]). D: The cumulative incidence of serious adverse events for the CLOP-CLOP group up to 52 weeks (16.7% [95% CI, 11.7% to 21.8%]). E: The cumulative incidence of vascular events of cerebral infarction, MI or other CV death for the CLOP-CLOP group up to 52 weeks (0.5% [95% CI, 0.0% to 1.4%]). F: The cumulative incidence of vascular events of cerebral infarction, MI, other CV death or hospitalization due to an ischemic event for the CLOP-CLOP group up to 52 weeks (4.3% [95% CI, 1.6% to 7.1%]).
