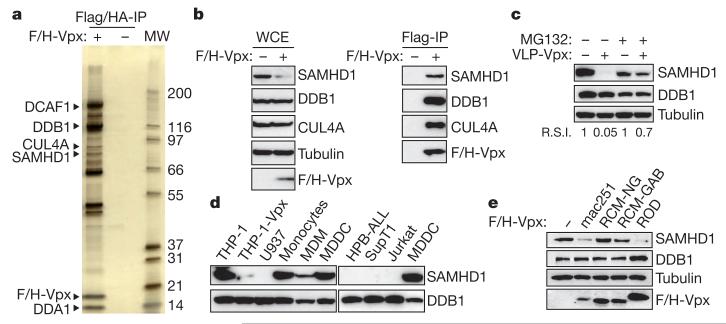
Figure 1. SAMHD1 interacts with Vpx and is degraded by the proteasome.
a, SIVmac251 Vpx was tandem-affinity-purified from Flag- and HA-tagged Vpxmac251 (F/H-Vpx)-expressing THP-1 cells (THP-1-Vpx) and peptide-eluted under native conditions. Eluates were separated on SDS–PAGE and silver stained. SAMHD1 and major, previously described, Vpxmac251 interactants identified using tandem mass spectrometry are indicated (MW, protein molecular weight marker in kDa). b, Whole-cell extract (WCE) and Flag-immunoprecipitated F/H-Vpx analysis by western blot against DDB1, CUL4A and SAMHD1. c, THP-1 cells were treated with 50 μM MG132 for 2 h before a 2-h incubation with Vpxmac251 containing virus-like particles (VLP-Vpx). After a further overnight incubation with MG132, whole-cell extracts were prepared and analysed by western blot with the indicated antibodies. R.S.I., relative signal intensity. d, Analysis of the expression profile of SAMHD1 in different cell types by western blot. e, THP-1 cells were transduced with a bicistronic retroviral vector allowing expression of Vpxmac251, VpxROD, VpxRCM-NG and VpxRCM-GAB and a selectable marker. After cell sorting and whole-cell extraction, the ability of Vpx variants to degrade SAMHD1 in THP-1 whole-cell extract was analysed by western blot using the indicated antibodies.