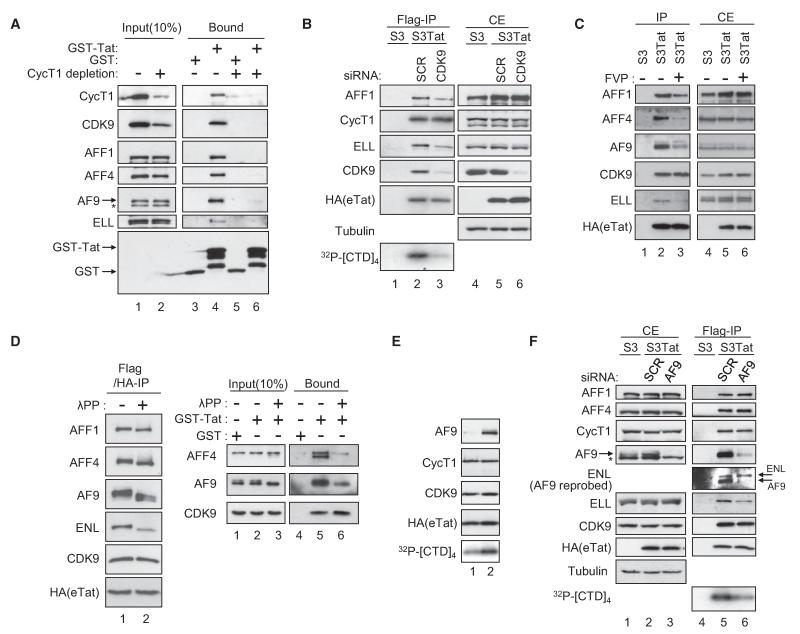
Figure 5. Assembly and Activity of HIV-1 Tat Complexes.
(A) CycT1 is required for Tatcom1 formation. Gst and Gst-Tat were incubated with mock- or CycT1-depleted HeLa nuclear extracts for 2 hr at 4 °C. Input and bound materials were analyzed by WB.
(B) CDK9 is required for Tatcom1 formation. Extracts of S3 or S3Tat that had been transfected with siRNA against CDK9 or a control (SCR) were subjected to Flag affinity chromatography. The presence of AFF1, ELL, CycT1, CDK9, and eTat in Flag IPs was analyzed by WB. CTD-kinase activity associated with Flag peptide eluates was assessed as in Figure 2E.
(C) S3Tat cells were exposed to 100 nM flavopiridol (FVP) for 20 hr or left untreated prior to whole-cell extraction and Flag IP. Eluates (IP) and cell extracts (CE) were analyzed by WB.
(D) Flag- and HA-purified eTat was either mock treated or incubated with 800 units lambda phosphatase (λPP) for 30 min and probed with the indicated antibodies (left panel). λPP-treated HeLa nuclear extracts were subjected to Gst pull-down using Gst-Tat or Gst and analyzed by WB.
(E) Fractions 5 and 7 from the glycerol gradient shown in Figure S2 were analyzed by WB. CTD-kinase activity associated with these fractions was assessed as in (C).
(F) AF9 potentiates CDK9 kinase activity. Extracts of S3Tat treated with AF9 or scrambled siRNA were subjected to Flag IP. Cell extracts (CE) and Flag-purified eTat were analyzed by WB, and eTat-associated kinase activity was tested as in (C).