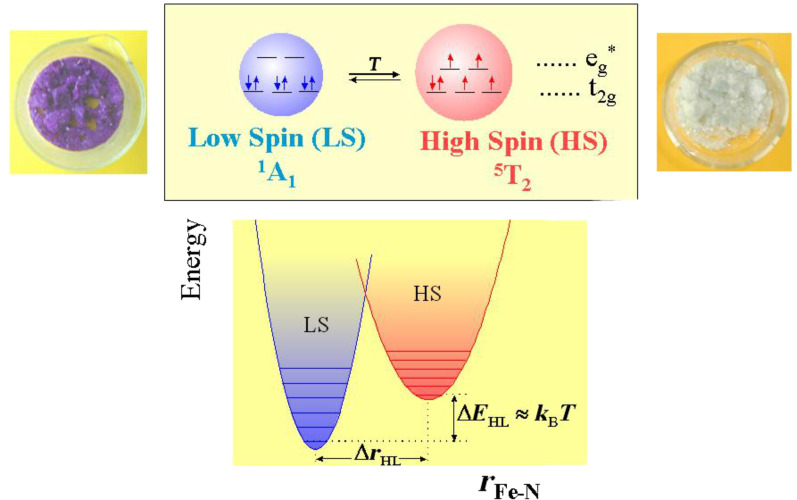
Figure 1.
Change of electron distribution between HS and LS states of an octahedral iron(II) coordination compound exhibiting thermal spin crossover. The orbitals eg* and t2g arise from splitting of the 3d orbitals in an octahedral ligand field. Depletion of charge in the antibonding eg* orbitals during HS to LS transition shortens the metal-to-ligand bond distances and reduces the molecular volume. SCO is mostly accompanied by a color change, e.g., in the present case, from white in the HS state to violet in the LS state. The condition to fulfil in order to observe thermal spin crossover is ΔEHL ≈ kBT. Increasing the temperature favors the HS state, decreasing it favors the LS state [23]. (Reproduced with permission from [36]. Copyright 2012 Wiley-VCH Verlag GmbH & Co. KGaA).