Fig. 2.
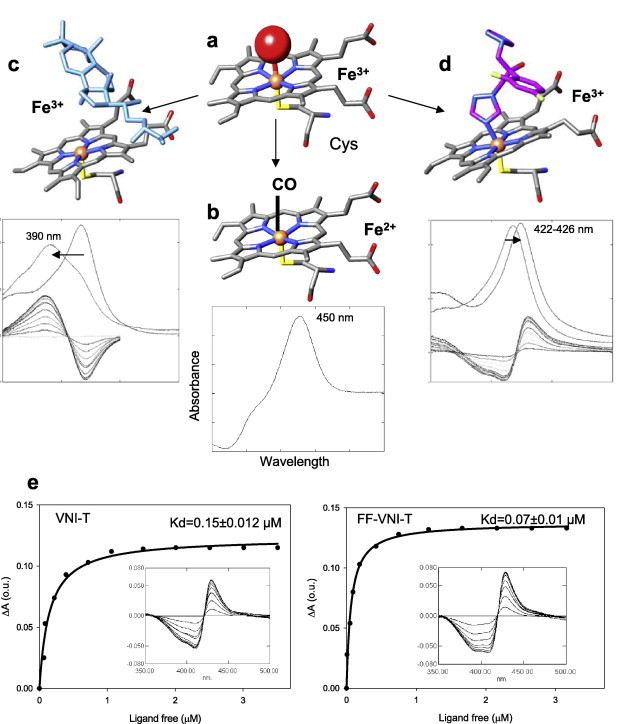
P450 spectral responses to ligand binding. (a) In the ferric (Fe3+) state CYP51 contains a water molecule (red sphere) coordinated to the heme iron (orange sphere); low-spin hexacoordinated state, the Soret band maximum is at 417 nm. (b) When the iron is reduced (Fe2+) and binds CO, the Soret band maximum shifts to ∼450 nm. (c) When the substrate (eburicol is shown in light blue) binds to the enzyme, it displaces water from the iron coordination sphere; Fe3+ becomes pentacoordinated high-spin, the Soret band maximum shifts to the left (394 nm), producing type 1 spectral response. (d) When a ligand stronger than water directly coordinates to the heme iron (carbon atoms of fluconazole are colored in pink) the Soret band maximum shifts further to the right, producing type 2 spectral response. (e) Spectral responses of T. cruzi CYP51 to two VNI derivatives, VNI-T and FF-VNI-T, and the corresponding titration curves. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
