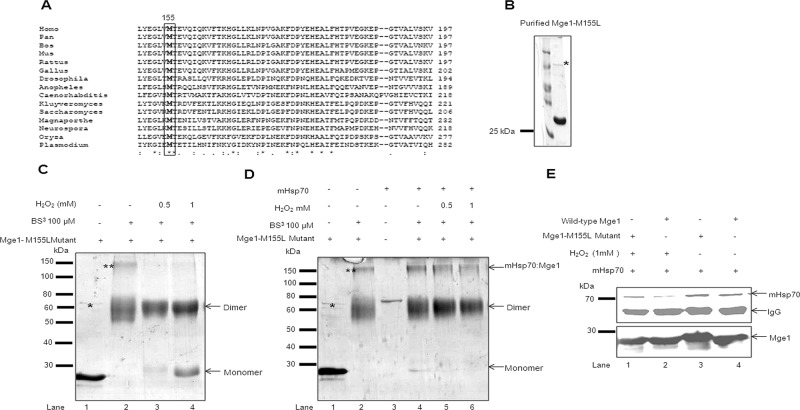
FIGURE 2:
Mge1-M155L mutant is resistant to H2O2-induced oxidative stress. (A) A multiple sequence alignment of C-terminal of Mge1 (149–197) across eukaryotic species. Black-shaded box represents the conserved methionine amino acid. (B) Purified recombinant Mge1 (M155L-Mge1) was resolved on SDS–PAGE and Coomassie stained. (C) Purified Mge1-M155L was preincubated in 20 mM sodium phosphate (pH 7.4) buffer in the presence (lanes 3 and 4) or absence (lanes 1 and 2) of H2O2 for 20 min, followed by cross-linking with BS3. (D) Purified Mge1-M155L was preincubated in 20 mM sodium phosphate (pH 7.4) buffer in the presence (lanes 3–5) or absence (lanes 1) of H2O2 for 20 min before incubation with mHsp70 (lanes 2–5) for 10 min. Then the samples were cross-linked with BS3 final concentration at 100 μM. Samples were incubated at room temperature for 30 min and quenched by Tris-HCl buffer. The samples were resolved on SDS–PAGE and Coomassie stained. (E) Purified wild type (lanes 2 and 4) and Mge1-M155L (lanes 1 and 3) were treated with 1 mM H2O2 for 20 min, followed by incubation with mHsp70 for 10 min. Then the samples were immunoprecipitated with anti Mge1 antibodies and probed with antibodies specific for mHsp70 (top) and Mge1 (bottom). This blot is representative of two independent experiments. Asterisk, the copurified DnaK with recombinant yeast Mge1-M155L; double asterisks, the DnaK–Mge1-M155L complex.