FIGURE 1:
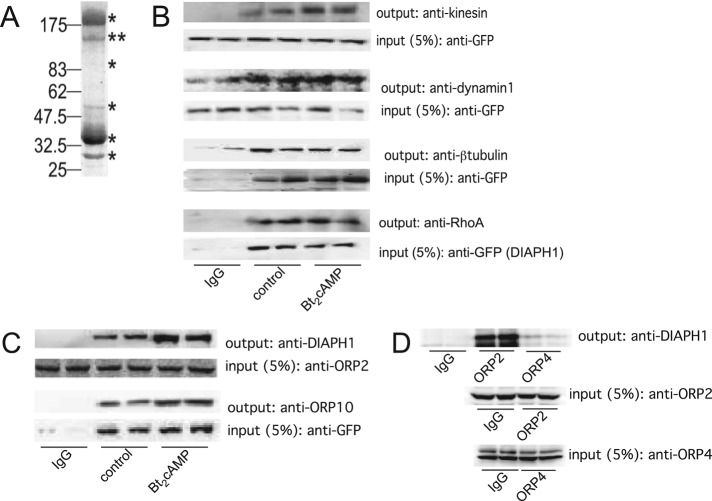
(A) Representative gel of H295R cell lysates that were immunoprecipitated using an anti-DIAPH1 antibody and subjected to SDS–PAGE and Coomassie staining. Immunoprecipitation and mass spectrometric analysis was performed on three separate occasions. Asterisks denote bands that were excised for mass spectrometry. (B) GFP-tagged or endogenous DIAPH1 was immunoprecipitated from control or Bt2cAMP-treated (0.4 mM, 30 min) samples and the immobilized proteins were washed, separated by SDS–PAGE, and analyzed by Western blotting using antibodies against kinesin (heavy chain), dynamin-1, β-tubulin, RhoA, or vimentin. Five percent of inputs were subjected to SDS–PAGE and Western blotting using an anti-GFP antibody. Blots shown are representative, and each immunoprecipitation was performed on at least three separate occasions, each time in duplicate. (C) Lysates from control or Bt2cAMP-treated H295R cells were immunoprecipitated using an anti-OPR2 antibody (input; second from top), and protein A/G agarose or H295R cells were transfected with pEGFP-DIAPH1 and control or Bt2cAMP-stimulated lysates immunoprecipitated using an anti-GFP antibody and protein A/G agarose (input; bottom). Immobilized proteins were washed, separated by SDS–PAGE, and analyzed by Western blotting using antibodies against DIAPH1 (output; top) or ORP10 (output, second from bottom). Immunoprecipitations were carried out three times, each time in duplicate. (D) H295R cells were plated onto 100-mm dishes and the lysates immunoprecipitated with 5 μg of rabbit IgG, anti-ORP2, or ORP4 and the purified proteins separated by SDS–PAGE. Western blotting was performed on 5% of the inputs for ORP2 (middle) or ORP4 (bottom) and the outputs for DIAPH1 (top). Shown are representative blots for studies that were carried out on three separate occasions, each time in duplicate.