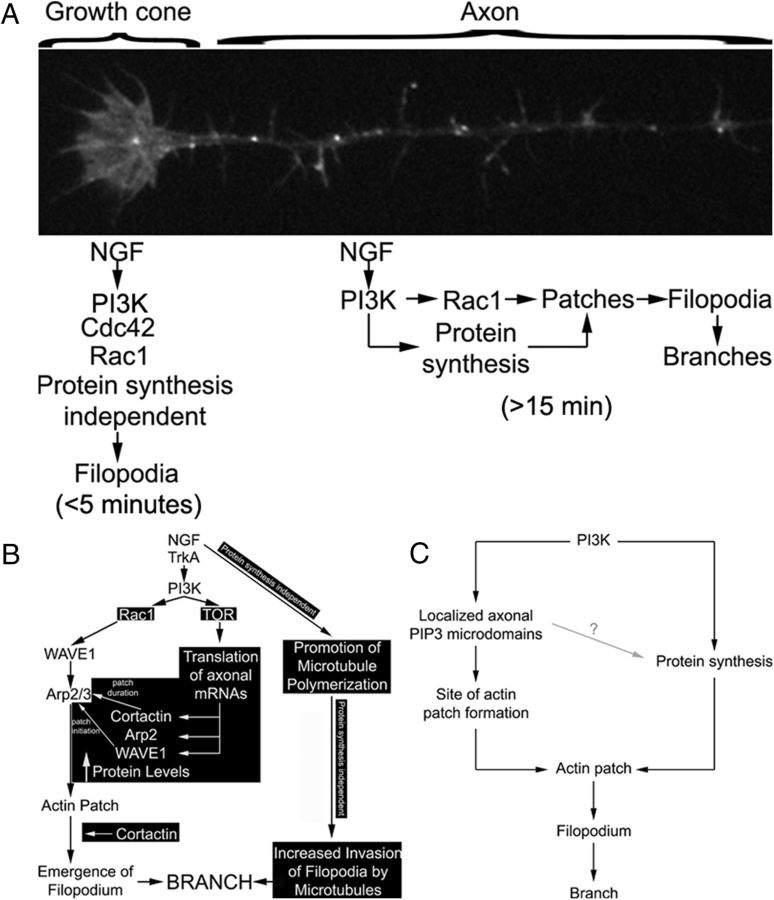
Figure 10.
Suggested model of the differential regulation of filopodia-mediated axon branch formation and the regulation of growth cone filopodia by NGF. A, The growth cones of chicken sensory neurons use both Cdc42 and Rac1 to control the number of growth cone filopodia in the presence of NGF, in a protein synthesis-independent manner. PI3K signaling at least partially drives the mechanism controlling the number of growth cone filopodia (Marsick et al., 2012). The response of growth cones to NGF is rapid and occurs maximally in <5 min. In contrast, in response to NGF-PI3K signaling, the axon shaft generates Rac1 and protein synthesis-dependent axonal filopodia, independent of Cdc42. Furthermore, the role for protein synthesis in this mechanism is reflected in the longer post-NGF treatment time required for the axon to mount a response (≥15 min). B, This study advanced our previous understanding of the mechanisms of sensory axon collateral branch formation in response to NGF. Previously established components of the mechanism are shown in black text, and the novel observations are denoted by white text on a black background. NGF activates PI3K, resulting in activation of Rac1, which drives the activity of the Arp2/3 activator WAVE1. WAVE1 activity promotes Arp2/3-dependent actin patch initiation. Cortactin positively regulates actin patch duration and contributes to the probability of emergence of a filopodium from the patch. PI3K activity concurrently also drives the intra-axonal synthesis of WAVE1, cortactin, and Arp2 from axonally targeted mRNAs. NGF, through as of yet undetermined signaling mechanisms, also promotes the polymerization of microtubules in axons and increases the localization of microtubule tips into filopodia. While NGF treatment increases the rate of actin patch formation, and thus filopodia, patches formed in the presence of NGF are not distinguishable from patches that form in the absence of NGF (Ketschek and Gallo, 2010). We propose that the axonal protein synthesis-dependent increases in WAVE1, cortactin, and Arp2 induced by NGF serve to provide additional building blocks for the generation of more actin patches, while maintaining the duration and probability of filopodial emergence from patches at pre-NGF levels. C, We propose that PI3K signaling has two major roles in the formation of axonal actin patches. Localized axonal microdomains of PIP3 accumulation driven by PI3K activity determine the sites of actin patch formation (Ketschek and Gallo, 2010). PI3K signaling, by driving axonal protein synthesis, also increases the axonal levels of the molecular machinery required to form more patches in the presence of NGF (e.g., WAVE1, cortactin, and Arp2). Whether PIP3 microdomains also locally drive protein synthesis is currently under investigation (gray line). The role of intra-axonal protein synthesis in this system may thus be to serve as a matching mechanism that provides cellular components required to maintain the system at steady state in the face of increased levels of signaling and the demand for increased output (i.e., increased formation of filopodia and branches). The role of intra-axonal protein synthesis in this system fits broadly under the umbrella of merotrophism as proposed by Alvarez et al. (2000).