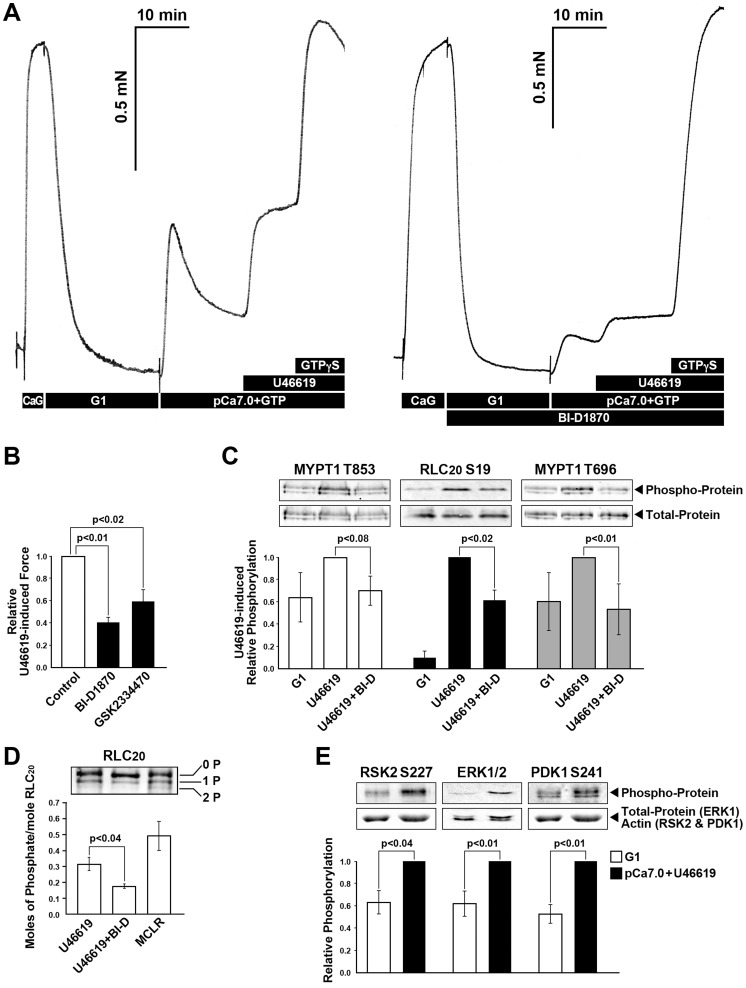
Figure 6. RSK and MEK/ERK signaling pathways contribute to TXA2-induced Ca2+ sensitized force and MYPT1 and RLC20 phosphorylation in α-toxin permeabilized rabbit pulmonary artery SM.
(A) U46619 (300 nM) induced Ca2+ sensitized force is suppressed in the presence of RSK inhibitor, BI-D1870 (100 nM). Subsequent addition of GTPγS, as a measure of the remaining component of Ca2+ sensitized force, reached the same magnitude of force but was proportionately greater in the presence of the RSK inhibitor BI-D1870 than in its absence. (B) Summary of panel A along with an effect of PDK1 kinase inhibitor GSK2334470 (30 µM) on U46619-induced force. (C) RSK inhibitor, BI-D1870 (1 µM) significantly inhibited the U46619 (300 nM) increased phosphorylation of MYPT1 Thr853 (n = 3), RLC20 phosphorylation at Ser19 (n = 5) and MYPT1 Thr696 phosphorylation (n = 5). (D) Unphosphorylated (0P), singly (1P) and doubly phosphorylated (2P) RLC20 from SM samples stimulated with U46619 with and without BI-D1870 as in panel C were separated on urea gels. A positive control for doubly phosphorylated RLC20 was a β-escin treated SM stimulated with microcystin. (E) U46619 stimulation increased the phosphorylation of proteins that regulate RSK activation; RSK2 Ser227 (n = 8), ERK1/2 (n = 7) and PDK1 Ser241(n = 15).