Abstract
The 5-lipoxygenase (5LO) enzyme is widely distributed within the central nervous system. Previous works showed that this protein is up-regulated in Alzheimer’s disease (AD), and that its genetic absence results in a reduction of Amyloid beta (Aβ) levels in the Tg2576 mice. However, its contribution to tau pathology remains to be investigated. To this end we studied the effect of 5LO chronic pharmacologic inhibition on endogenous tau level and metabolism in the same mice. The phosphorylation of tau at S396 and S396/404 in the brains of mice receiving zileuton, a selective and specific 5LO inhibitor, was significantly reduced when compared with their controls, while there was no significant change of tau phosphorylation at S202/T205, T231/S235 and T181 epitopes. The 5LO-dependent reduction of tau phosphorylation resulted from a significant decrease in the level and activity of the cyclin-dependent kinase-5 but not other kinases. Our findings highlight the novel functional role that neuronal 5LO plays in modulating tau phosphorylation, and suggest that pharmacological inhibition of 5LO could provide a novel therapeutic opportunity also for AD-related tau pathology.
Keywords: Alzheimer’s disease, transgenic mouse models, tau protein, beta amyloid, 5Lipoxygenase
1. Introduction
Alzheimer’s disease (AD) is a progressive neurodegenerative disease characterized by two major pathological hallmark lesions: extracellular accumulation of β-amyloid (Aβ) plaques and intracellular accumulation of insoluble microtubule-associated protein tau as neurofibrillary tangles (NFTs)(Querfurth and Laferla, 2010). Mutation in the tau gene have been linked to NFT formation in several neurodegenerative diseases called tauopathies in which no Aβ deposits are detected (Iqbal et al., 2010; Mederios et al., 2011). However, no mutations have been found in the tau gene in AD, and the relationship between tau metabolic fate, amyloid deposition and neurodegeneration is difficult to establish.
Recently, we showed that the enzyme 5-Lipoxygenase (5LO) is a new active player in AD pathogenesis. Thus, its protein levels are significantly increased in AD post-mortem brain tissues and its genetic absence or pharmacological blockade both result in a significant reduction in the Aβ levels and deposition of a transgenic mouse model of AD-like amyloidosis, i.e. Tg2576 mice (Firuzi et al., 2008; Chu and Pratico 2011; Chu et al., 2012).
Because recent evidence indicates, at least in AD animal models, a link between Aβ and tau pathology and suggests that Aβ accumulation can exacerbate tau pathology (Oddo et al., 2008; Robertson et al., 2007), we were interested to assess whether pharmacological inhibition of 5LO activation would results in significant changes in tau levels and/or metabolism.
To this end, Tg2576 mice were chronically administered with zileuton, a selective 5LO inhibitor (Berger et al., 2007), and the effect on tau levels and metabolism assessed.
2. Materials and Methods
2.1. Mice and treatments
All animal procedures were approved by the Institutional Animal Care and Usage Committee, and in accordance with the National Institute of Health guidelines. Female Tg2576 transgenic mice expressing human APP with the Swedish mutation (K670N/M671L) were used in these studies. They were genotyped by polymerase chain reaction (PCR) analysis using tail DNA and kept in a pathogen-free environment, on a 12-hour light/dark cycle and had access to food and water ad libitum. Starting at 7months of age, mice were randomized to receive zileuton (200mg/L) (n = 10) or vehicle (n = 8) in their drinking water for 8 months until they were 15-month-old. Considering that each mouse drinks in average 3–4 ml/day of water, the final concentration of the active drug was approximately 0.6–0.8mg/day. During the study, animals gained weight regularly, and no significant difference in weight was detected between the two groups. No macroscopic effect on the overall general health was observed in the animals receiving the active treatment. After sacrifice, animals were perfused with ice-cold 0.9% Phosphate Buffered saline (PBS), brain removed and dissected in two hemihalves by midsagittal dissection. One was immediately stored at −80°C for Western blot analyses and kinase activity assay, and the other immediately immersed in 4% paraformaldehyde in 0.1 M PBS (pH 7.6) overnight for immunohistochemistry studies.
2.2. Immunohistochemistry
Immunostaining was performed as previously reported by our group (Chu et al., 2012; Yang et al., 2010; Joshi et al., 2012). Briefly, serial 6-μm-thick coronal sections were mounted on 3-aminopropyl triethoxysilane (APES)-coated slides. Every eighth section from the habenular to the posterior commissure (8–10 sections per animal) was examined using unbiased stereological principles. The sections were deparaffinized, hydrated, treated with 3% H2O2 in methanol and subsequently retrieve antigen with sodium citrate buffer. Sections were blocked in 2% fetal bovine serum before incubation with primary antibodies: anti total tau, PHF1 and PHF13 overnight at 4°C (Table). Subsequently, sections were incubated with biotinylated anti-mouse IgG (Vector Lab., Burlingame, CA, USA) and then developed by using the avidin-biotin complex method (Vector Lab.) with 3, 3′-diaminobenzidine (DAB) as a chromogen. Light microscopic images were used to calculate the Integrated Optical Density (IOD) the obtained immunoreactivity using the software Image-Pro Plus for Windows version 5.0 (Media Cybernetics) (Chu et al., 2012; Yang et al., 2010; Joshi et al., 2012).
Table.
Antibodies used in the study.
| Antibody | Immunogen | Host | Application | Source |
|---|---|---|---|---|
| Tau | Purified denatured bovine MAP | Mouse | WB, IHC, IF | Millipore |
| AT-8 | Peptide containing phospho-S202/T205 | Mouse | WB | Pierce |
| AT-180 | Peptide containing phospho-T231/S235 | Mouse | WB | Pierce |
| AT-270 | Peptide containing phospho-T181 | Mouse | WB | Pierce |
| PHF-13 | Peptide containing phospho-Ser396 | Mouse | WB, IHC | Cell Signaling |
| PHF-1 | Peptide containing phospho-Ser396/S404 | Mouse | WB, IHC | Dr. P. Davies |
| GSK3α/β | aa 1–420 full length GSK-3β of Xenopus origin | Mouse | WB, IHC, IF, IP | Millipore |
| p-GSK3α/β | aa around Ser21 of human GSK-3a | Rabbit | WB, IP | Cell Signaling |
| JNK2 | aa of human JNK2 | Rabbit | WB | Cell Signaling |
| SAPK/JNK | aa of recombinant human JNK2 fusion protein | Rabbit | WB | Cell Signaling |
| Phospho-SAPK/JNK | aa Thr183/Tyr185 of human SAPK/JNK | Mouse | WB | Cell Signaling |
| Cdk5 | aa C-terminus of Cdk5 of human origin | Rabbit | WB, IF | Santa Cruz |
| P35/P25 | aa C-terminus of p35/25 of human origin | Rabbit | WB, IF | Santa Cruz |
| PP-2A | A 16 residue synthetic peptide corresponding to aa 295–309 of the 36 kDa catalytic subunit of human protein phosphatase 2A(PP2A) | Mouse | WB, IP, IC | Millipore |
| GFAP | Spinal cord homogenates of bovine origin | Mouse | WB | Santa Cruz |
| CD45 | Mouse thymus or spleen | Rat | WB | BD Pharmigen |
| Actin | aa C-terminus of Actin of human origin | Goat | WB | Santa Cruz |
2.3. Western blot analyses
Brain tissues were homogenized and extracted in RIPA buffer containing proteases and phosphatases inhibitors, then the extracts used for western blot analyses, as previously described (Chu et al., 2012; Yang et al., 2010; Joshi et al., 2012). Samples were electrophoresed on 10% Bis–Tris gels or 3–8% Tris–acetate gel (Bio-Rad, Richmond, CA, USA), according to the molecular weight of the target molecule, transferred onto nitrocellulose membranes (Bio-Rad), and then incubated with appropriate primary antibodies shown in the Table. After three washings with T-TBS, membranes were incubated with IRDye 800CW or IRDye 680CW-labeled secondary antibodies (LI-COR Bioscience, NE, USA) at 22°C for 1 h. Signals were developed with Odyssey Infrared Imaging Systems (LI-COR Bioscience). Beta-actin was always used as internal loading control.
2.4. Cyclin-dependent kinase 5 activity assay
For determination of cyclin-dependent kinase (cdk)-5 kinase activity, the cells were rinsed with PBS and lysed in buffer A (50 mM Tris–HCl [pH 8.0], 150 Mm sodium chloride, 1% NP-40, 0.5% sodium deoxycholate, 0.1% sodium dodecyl sulfate, 0.02% sodium azide and freshly added protease inhibitors [100 μg/ml phenylmethysulfonyl fluoride and 1 μg/ml Aprotinin]). Following incubation on ice for 0.5 h, the samples were centrifuged at 12,000g at 4 °C for 20 min and supernatants collected. The supernatant (300 μl equivalent to 150 μg protein) was incubated with 3 μg of anti-cdk5 antibody (Santa Cruz, CA, USA) at 4 °C for 2 h. Protein A agarose beads (50 μl) were then added and incubated for another hour. The immunoprecipitates were washed with lysis buffer three times and once with HBS buffer (10 mM HEPES, pH 7.4, 150 mM NaCl). The kinase activity of the immunoprecipitated cdk5 was determined by using histone H1 (Santa Cruz, CA, USA). Beads were incubated with 5 μg of histone H1 (Santa Cruz, CA, USA) in HBS (20 μl) containing 15 mM MgCl2, 50 μM ATP, 1 mM dithiothreitol, and 1 μCi of [32P] ATP. After 30 min of incubation at 30 °C, the reaction products were determined by a liquid scintillation counter (Perkin Elmer LAS, model 1209-005).
2.5. Cell cultures
N2A (neuro-2 A neuroblastoma) cells stably expressing human APP carrying the K670 N, M671 L Swedish mutation (APP swe) were grown in Dulbecco’s modified Eagle medium supplemented with 10% fetal bovine serum, 100 U/ml penicillin, 100 μg/ml streptomycin (Cellgro, Herdon, VA, USA), and 400 μg/ml G418 (Invitrogen, Carlsbad, CA), at 37°C in the pre sence of 5 % CO2 as previously described (Chu and Pratico, 2011). For each experiment, equal numbers of cells were plated in six-well plates, 24hr later media were removed and fresh media containing either Zileuton or vehicle were added. After 24 hours incubation cell pellets harvested in lytic buffer for immunoblot analyses as described in the previous paragraphs.
2.6. Data analysis
Data analyses were performed using GraphPad Prism 5.0. Statistical comparisons were performed by Unpaired Student’s t-test. All data are presented as mean ± S.E.M. Significance was set at p < 0.05.
3. Results
3.1. In vivo studies
Pharmacological blockade of 5LO reduces brain tau phosphorylation
Starting at seven months of age Tg2576 mice were randomized to receive zileuton (200mg/L) or vehicle for 8 months, then sacrificed and the effect of the treatment on tau level and metabolism assessed.
At the end of the study we observed that compared with controls, mice receiving zileuton had no change in their brain levels of total tau (Figure 1A, B). By contrast, we found that the same animals manifested a significant reduction in its phosphorylated forms at S396 and S396/S404 as recognized by the antibody PHF13 and PHF1 respectively, which are typically markers of a late-stage pathological tau accumulation (Figure 1A, B). No significant changes were observed in other phopshorylation epitopes such as AT8, AT180 and AT270, which are considered early stage phosphorylation changes (Figure 1A, B). Consistent with the immunoblot analysis results, immunohistochemical staining demonstrated a significant decrease in the somatodendritic accumulation of phosphorylated epitopes as recognized by PHF13 and PHF-1 positive immunoreactivity in the brains of mice receiving zileuton (Figure 1C, D).
Figure 1. Chronic administration of zileuton decreases tau phosphorylation in the brains of Tg2576 mice.
A. Representative western blots of total tau (Tau), phosphorylated tau as recognized by PHF13, AT8, AT180, AT270, and PHF-1 antibodies in brain homogenates from mice receiving zileuton or placebo. B. Densitometric analyses of the immunoreactivities to the antibodies shown in the previous panel (*p <0.05) (n=8 placebo, 10 zileuton). C. Representative immunohistochemical staining for tau, PHF13 and PHF-1 positive areas in brain sections of mice receiving zileuton or placebo. D. Densitometric analyses of the immunoreactivities to the antibodies shown in the previous panel (n=4 per group)(*p <0.01). Values represent mean ± SEM.
5LO regulates tau phosphorylation by modulating Cdk5 kinase
It is known that tau protein is regulated by an array of post-translational modifications, which includes phosphorylation changes controlled by different kinases. To investigate the molecular mechanism involved in the effect of zileuton treatment on tau phosphorylation in mice brains, we examined some of the kinases which are considered major regulators of this protein post-translational modification. As shown in figure 2A, no differences in the levels of total or phosphorylated GSK-3α and GSK-3β, JNK2, and total and phosphorylated SAPK/JNK were observed between the two groups of mice. By contrast, we found that brains of Tg2576 mice receiving zileuton had a significant reduction in the activation state of the cyclin-dependent kinase (cdk5), as evaluated by analyzing levels of p35 and p25, its co-activators, which were significantly decreased (Figure 2A, B). This finding was further confirmed by directly assaying for the kinase activity in the same tissues where we show that compared with controls the cdk5 in vitro activity was significantly decreased in the brains of mice treated with zileuton (Figure 2C). Finally, no significant changes between the two groups were also observed for protein phosphatase (PP)-2A, an enzyme which has been involved in dephosphorylating tau (Figure 2A).
Figure 2. Chronic administration of zileuton modulates brain tau metabolism via the cdk5 kinase in Tg2576 mice.
A. Representative western blot analyses of GSK3α, GSK3β, p-GSK-3α, p-GSK-3β, JNK2, SAPK/JNK, p-JNK2/3, p-JNK1, cdk-5, p35 and p25 in brain homogenates from mice receiving zileuton or placebo. B. Densitometric analyses of the immunoreactivities to the antibodies shown in the previous panel (*p <0.05). C. Cdk5 kinase activity in brain homogenates from mice receiving zileuton or placebo (n=8 placebo, 10 zileuton) (*p <0.01). Values represent mean ± SEM.
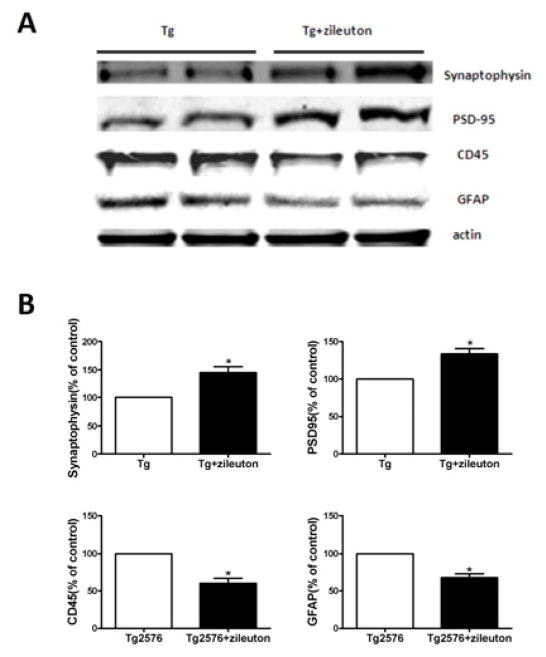
Pharmacological blockade of 5LO modulates synaptic integrity
Since tau pathology has been correlated with the severity of dementia and memory impairments for which synaptic integrity is an important factor, next we investigated whether pharmacological blockade of 5LO had any effect on this aspect of the AD-like phenotype. As shown in figure 3, we observed that compared with controls steady state levels of two distinct synaptic proteins synaptophysin and post-synaptic protein-95 (PDS-95) were significantly increased in mice receiving zileuton. Additionally we observed that compared with controls, mice receiving zileuton had biochemical evidence for a significant reduction of GFAP as well as CD45, markers of astrocytes and microglia activation respectively (figure 3).
Figure 3. Chronic administration of zileuton prevents synaptic dysfunction in Tg2576 mice.
A Representative western blot analyses of synaptophysin and protein post synaptic-95 (PDS-95), GFAP and CD45 in brain homogenates from mice receiving zileuton or placebo. B. Densitometric analyses of the immunoreactivities to the antibodies shown in the previous panel (n=4 per group) (*<0.05). Values represent mean ± SEM.
3.2. In vitro studies
Zileuton influences tau phosphorylation in a cdk-5-dependent manner
To gain further support and confirm our observation in the Tg2576 mice, next we set up a series of in vitro experiments. N2A-APPswe cells were incubated with Zileuton for 24 hr at different concentration (1, 10, 25, 50, 100 μM) or vehicle. At the end of this period, cell lysates were collected and immunoblotted for levels of total tau and its phosphorylation forms. As shown in figure 4, we found that there were not significant changes in levels of total tau between cells incubated with vehicle or zileuton. By contrast, in the presence of zileuton cells has a statistically significant reduction in tau phosphorylation at epitopes S396 and S396/S404 as recognized by the antibodies PHF1 and PFH13 (Figure 4A, B). In addition, we observed that the same treatment resulted in a significant reduction in the steady state levels of p25 and p35 but not cdk5 (Figure 4A, B), which, similar to the ex vivo experiments, was accompanied by a reduction in its in vitro activity (not shown).
Figure 4. Pharmacological blockade of 5lipoxygenase modulates tau phosphorylation via the cdk5 kinase in neuronal cells.
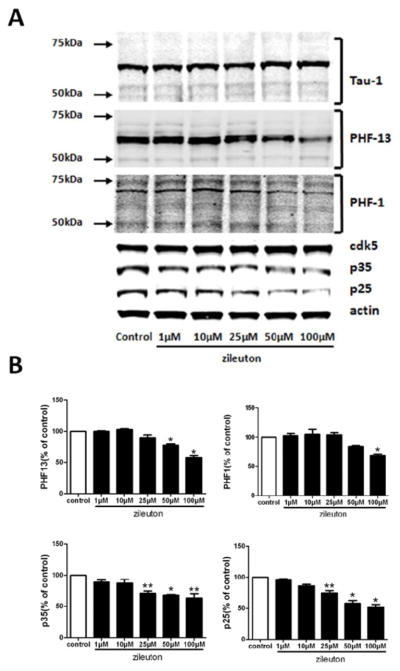
N2A-APPswe cells were incubated with increasing concentration of zileuton or vehicle (control) for 24 hours and cell lysates collected. A. Representative western blots of tau, phosphorylated tau as recognized by the antibody PHF13, AT8, AT180, AT270, PHF-1, cdk-5, p23 and p25 in the lysates of zileuton or vehicle-treated (control) cells. B. Densitometric analyses of the immunoreactivities to the antibodies shown in the previous panel (n = 3 per each condition, **p < 0.05, *p< 0.01). Values represent mean ± SEM
4. Discussion
In the present study we provide the first experimental evidence supporting the concept that 5LO pharmacological blockade could be a novel therapeutic intervention in AD tauopathy by reducing the hyperphosphorylation status of endogenous tau in a mouse model of the disease.
A constant feature of AD brain pathology is the intracellular accumulation of insoluble microtubules associated protein tau in selected brain regions. This type of brain pathology is also present in other neurodegenerative diseases collectively referred to as tauopathies, which include progressive supranuclear palsy, Pick’s disease and corticobasal degeneration (Hernadez et al., 2007). Today we know that in these conditions tau because of an excessive phosphorylation acquires a pathological conformation, detaches from the microtubules ad accumulates as neurofibrillary tangles. However, the mechanisms involved in these pathological modifications are still unknown. Emerging evidence indicates that a part form the tauopathies secondary to mutations in the tau gene, in the vast majority of the cases tau pathology is probably the results of the interaction between environmental elements and genetic risk factors (Geschwind 2003). In recent years, our lab has been very interested in the 5LO enzymatic pathways as a novel active player in AD pathogenesis. Thus, we showed that changes in its expression levels (genetic absence, over-expression or its pharmacological inhibition) result in a modulation of the brain amyloidotic phenotype of a transgenic mouse model of AD, Tg2576 mice (Firuzi et al., 2008; Chu and Pratico, 2011; Chu et al., 2012). However, no data are available on the effect that pharmacological inhibition of this pathway might have on endogenous tau levels and metabolism in the same animal model. In the current study, by using zileuton, an orally available selective and specific 5-LO inhibitor, we demonstrated that this therapeutic approach results in a significant reduction of brain tau phosphorylation at specific epitopes in the Tg2576 mice.
Thus, by using both a biochemical and immunohistochemical approach we found that mice receiving zileuton had a selective decrease in tau phosphorylation at Ser396 (detected by antibody PHF-13), and Ser396/404 (detected by antibody PHF-1). By contrast, we did not find any significant differences when other phoshoepitopes, as recognized by the antibodies AT8, AT180 and AT270, were assayed. Interestingly, previous work showed that at least in AD animal models the latter represent early markers of tau phosphorylation, whereas the PHF1 and PHF-13 reactivity represents middle and late stages (Oddo et al., 2003; Kitazawa et al., 2005), which is compatible with the age of our mice (i.e. 15month-old). In addition to those changes in tau phosphorylation, we also observed a significant increase in two main synaptic proteins suggesting a beneficial effect of 5LO pharmacologic blockade on synapse integrity. Interestingly this effect was also associated with a dramatic reduction in markers of gliosis, confirming the anti-inflammatory actions of the drug investigated.
Because of the known modulatory effect of 5LO on Aβ in vitro and in vivo, it is possible that the effect of zileuton on tau is somewhat secondary to the one we previously reported on Aβ (Chu and Pratico 2011). However, we recently provided experimental evidence showing that 5LO acts on tau metabolism independently from its effect on Aβ (Chu et al. 2012 a).
In an effort to elucidate the molecular mechanism whereby 5LO-inhibition induces a selective reduction of in vivo tau phosphorylation, we assayed several putative tau kinases. We measured the total and activated forms of GSK-3, JNK2 and SAPK/JNK since they have been implicated in regulating the phosphorylation status of tau (Lin et al., 2003; Maldonado et al., 2011). In the current study, we found that 5LO pharmacological inhibition did not alter the activation status of any of these kinases. Interestingly, we found that 5LO affected specifically the cdk5 kinase pathway whose activation is regulated by its binding to activators proteins p35 and p25, a cleaved product of p35 (Humbert et al., 2000; Lee et al., 2003). Thus, while we found no difference in the steady-state levels of cdk5 protein between the two groups of mice, we detected a significant decrease in the levels of p25 and p35 in mice receiving the active drug, suggesting that this kinase activation is responsible for the changes in tau phosphorylation in vivo.
To gather further experimental support on the role of 5LO on tau metabolism we embarked in a series of in vitro experiments. Neuronal cells incubated with zileuton had a dose-dependent and significant decrease in tau phosphorylated immunoreactivities as recognized by the antibody PHF-13 and PHF-1. Confirming the in vivo data, we also observed that the cdk5 kinase pathway was significantly reduced as shown by the selective decrease of its co-activator p25 and p35, and the measurement of cdk5 enzymatic activity in cells incubated with zileuton.
In summary, our current studies demonstrate a novel functional role for 5LO in modulating tau phosphorylation in vivo via the cdk-5 kinase pathway, and establish the 5-LO enzyme as a novel therapeutic target for AD-like tau pathology. Because this class of drug would target the two most important hallmark AD lesions (i.e., Aβ and tau), our discovery represents a strong biologic support for the hypothesis that pharmacological inhibition of this enzymatic pathway is a unique and viable therapeutic opportunity for AD. To this end, some concern could be legitimate considering the irreversible chronic blockade of 5LO activity by zileuton-like drugs and some side-effects could be anticipated for long-term usage of this type of drug. However, it is important to stress two important piece of information about zileuton. First, the drug has been FDA-approved for more than a decade now and widely prescribed for asthmatic conditions, so we have plenty of data on safety. Second, we have shown in the current study that the mice receiving the drug did not manifest any differences or changes when compared with the placebo group.
In conclusion, our findings highlight the novel functional role that neuronal 5LO plays in modulating tau phosphorylation, and suggest that pharmacological inhibition of 5LO could provide a novel therapeutic opportunity also for AD-related tau pathology.
Acknowledgments
This study was in part supported by US National Institute of Health (NIH) grants AG033568 and NS071096.
Footnotes
Disclosure Statement
The authors have no conflict of interest to disclose in relation to the work described in the present paper.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Querfurth HW, Laferla FM. Alzheimer’s disease. N Engl J Med. 2010;362:329–344. doi: 10.1056/NEJMra0909142. [DOI] [PubMed] [Google Scholar]
- 2.Iqbal K, Liu F, Gong CX, Grundke-Iqbal I. Tau in Alzheimer disease and related tauopathies. Curr Alz Res. 2010;7:656–664. doi: 10.2174/156720510793611592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mederios R, Baglietto-Vargas D, Laferla F. The role of tau in Alzheimer’s disease and related disorders. CNS Neurosci Therap. 2011;17:514–524. doi: 10.1111/j.1755-5949.2010.00177.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Firuzi O, Zhou J, Chinnici CM, Wisnieski T, Praticò D. Five-Lipoxygenase gene disruption reduces amyloid-{beta} pathology in a mouse model of Alzheimer’s disease. FASEB J. 2008;22:1169–1178. doi: 10.1096/fj.07-9131.com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chu J, Praticò D. Pharmacologic blockade of 5-Lipoxygenase improves the amyloidotic phenotype of an AD transgenic mouse model: involvement of γ-secretase. Am J Pathol. 2011;178:1762–1769. doi: 10.1016/j.ajpath.2010.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 6.Chu J, Giannopoulos PF, Ceballos-Diaz C, Golde TE, Praticò D. Adeno-associated virus brain deliver of 5-lipoxygenase modulates the AD-like phenotype of APP mice. Mol Neurodeg. 2012;5;7(1):1. doi: 10.1186/1750-1326-7-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chu J, Giannopoulos PF, Ceballos-Diaz C, Golde TE, Praticò D. 20125-Lipoxygenase gene transfer worsens memory, amyloid and tau brain pathologies in a mouse model of Alzheimer disease Ann Neurol. 72:442–454. doi: 10.1002/ana.23642.
- 8.Oddo S, Caccamo A, Tseng B, Cheng D, Vasilevko V, Cribbs D, et al. Blocking abeta 42 accumulation delays the onset and progression of tau pathology via the C terminus of the heat shock protein70-interacting protein: a mechanistic link between abeta and tau pathology. J Neurosci. 2008;28:12163–12175. doi: 10.1523/JNEUROSCI.2464-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Robertson ED, Scearce-Levie K, Palop JJ, Yan F, Cheng IH, Wu T, et al. Reducing endogenous tau ameliorates amyloid-beta-induced deficits in an Alzheimer’s disease mouse model. Science. 2007;16:750–754. doi: 10.1126/science.1141736. [DOI] [PubMed] [Google Scholar]
- 10.Berger W, De Chandt MTM, Cairns CB. Zileuton: clinical implications of 5-Lipoxyegnase inhibition in severe airways disease. Int J Clin Pract. 2007;61:663–676. doi: 10.1111/j.1742-1241.2007.01320.x. [DOI] [PubMed] [Google Scholar]
- 11.Yang H, Zhuo J, Chu J, Chinnici C, Praticò D. Amelioration of the Alzheimer’s disease phenotype by absence of 12/15-lipoxygenase. Biol Psych. 2010;68(10):922–929. doi: 10.1016/j.biopsych.2010.04.010. [DOI] [PubMed] [Google Scholar]
- 12.Joshi Y, Chu J, Praticò D. Stress hormone leads to memory deficits and altered tau phosphorylation in a mouse model of Alzheimer’s disease. J Alz Dis. 2012;31:167–176. doi: 10.3233/JAD-2012-120328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chu J, Praticò D. 5-Lipoxygenase as an endogenous modulator of amyloid-beta formation in vivo. Ann Neurol. 2011;69:34–46. doi: 10.1002/ana.22234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hernanadez F, Avila J. Tauopathies. Cell Mol Life Sci. 2003;64:2219–2233. doi: 10.1007/s00018-007-7220-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Geschwind DH. Tau phosphorylation, tangles, and neurodegeneration: the chicken or the egg? Neuron. 2003;40:457–460. doi: 10.1016/s0896-6273(03)00681-0. [DOI] [PubMed] [Google Scholar]
- 16.Oddo S, Caccamo A, Shepherd JD, Murphy MP, Golde TE, Kayed R, et al. Triple transgenic model of Alzheimer’s disease with plaques and tangles: intracellular Abeta and synaptic dysfunction. Neuron. 2003;39(3):409–421. doi: 10.1016/s0896-6273(03)00434-3. [DOI] [PubMed] [Google Scholar]
- 17.Kitazawa M, Oddo S, Yamasaki TR, Green KN, Laferla F. Lipopolysaccharide-induced inflammation exacerbates tau pathology by a cyclin-dependent kinase 5-mediated pathway in a transgenic model of Alzheimer’s disease. J Neurosci. 2005;25:8843–8853. doi: 10.1523/JNEUROSCI.2868-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu SJ, Zhang AH, Li HL, Wang Q, Deng HM, Netzer WJ, et al. Overactivation of glycogen synthase kinase-3 by inhibition of phosphoinositol-3 kinase and protein kinase C leads to hyperphosphorylation of tau and impairment of spatial memory. J Neurochem. 2003;87:1333–1344. doi: 10.1046/j.1471-4159.2003.02070.x. [DOI] [PubMed] [Google Scholar]
- 19.Maldonado H, Ramírez E, Utreras E, Pando ME, Kettlun AM, Chiong M, Kulkarni AB, Collados L, Puente J, Cartier L, Valenzuela MA. Inhibition of cyclin-dependent kinase 5 but not of glycogen synthase kinase 3-β prevents neurite retraction and tau hyperphosphorylation caused by secretable products of human T-cell leukemia virus type I-infected lymphocytes. J Neurosci Res. 2011;89:1489–1498. doi: 10.1002/jnr.22678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Humbert S, Dhavan R, Tsai L. P39 activates cdk5 in neurons, and is associated with the actin cytoskeleton. J Cell Sci. 2000;113:975–983. doi: 10.1242/jcs.113.6.975. [DOI] [PubMed] [Google Scholar]
- 21.Lee MS, Tsai LH. Cdk5: one of the links between senile plaques and neurofibrillary tangles? J Alz Dis. 2003;5:127–137. doi: 10.3233/jad-2003-5207. [DOI] [PubMed] [Google Scholar]