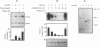Abstract
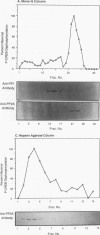
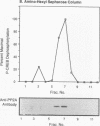
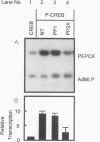
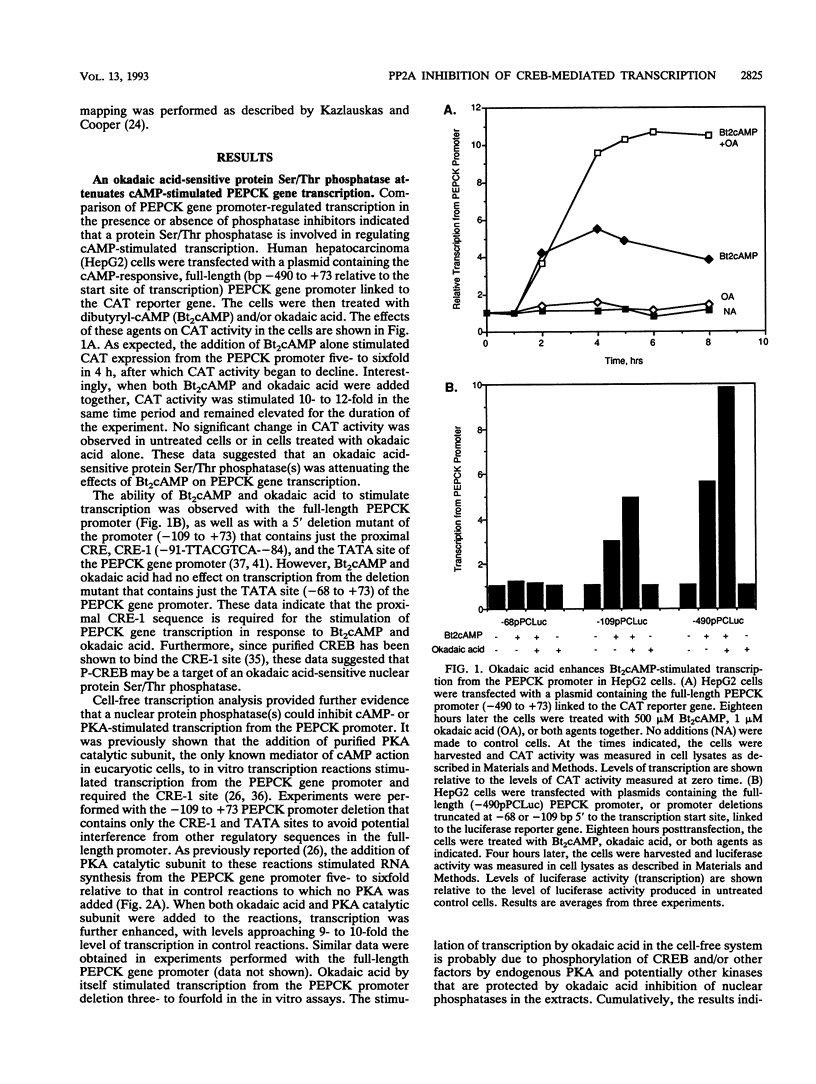
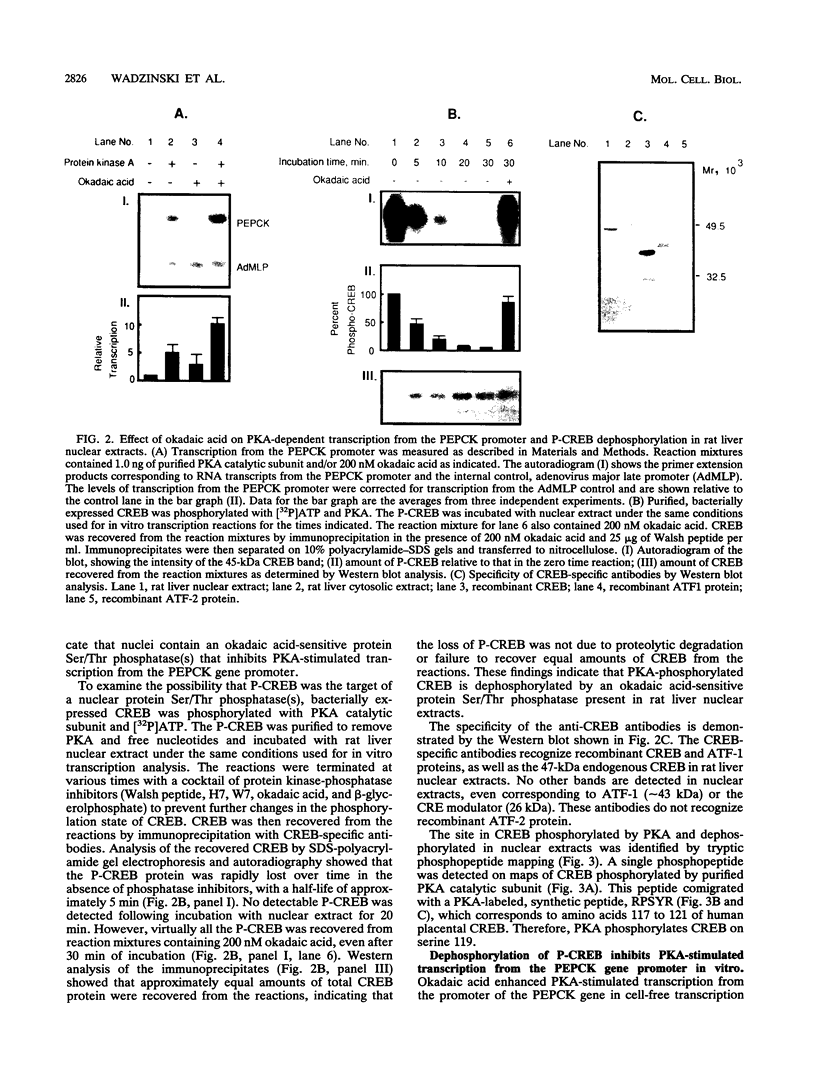
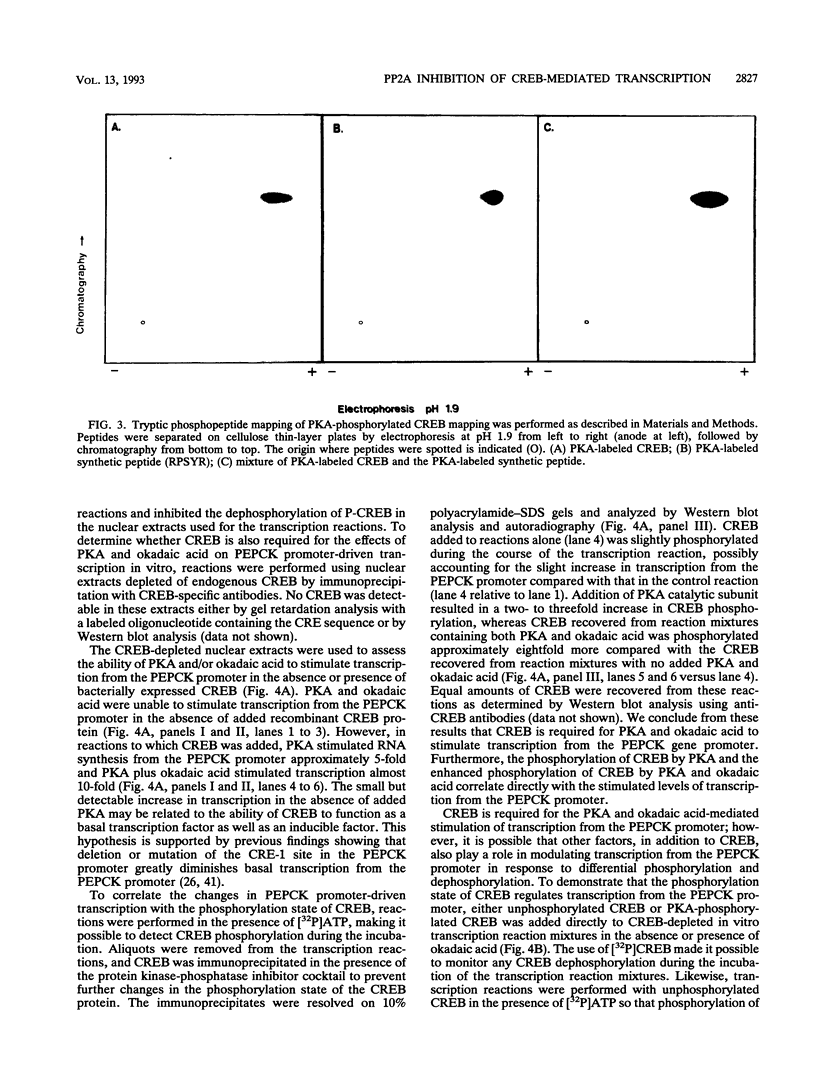
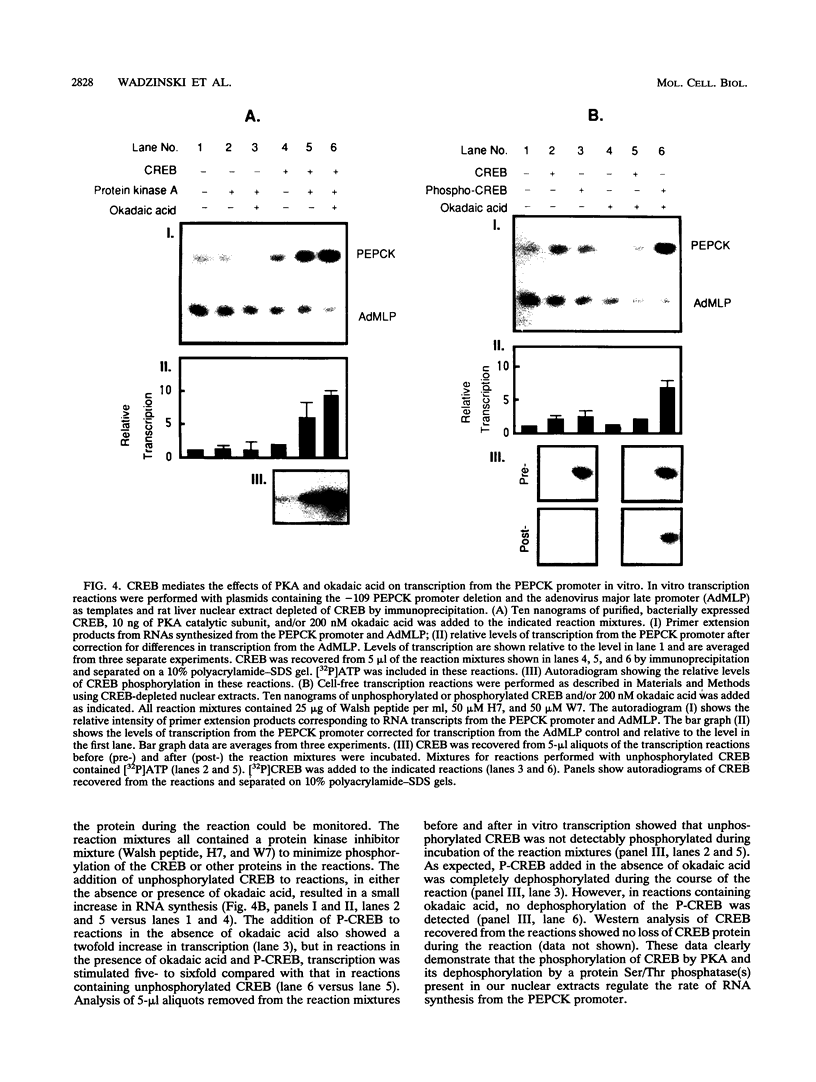
Cyclic AMP (cAMP)-dependent protein kinase A (PKA) stimulates the transcription of many eucaryotic genes by catalyzing the phosphorylation of the cAMP-regulatory element binding protein (CREB). Conversely, the attenuation or inhibition of cAMP-stimulated gene transcription would require the dephosphorylation of CREB by a nuclear protein phosphatase. In HepG2 cells treated with the protein serine/threonine (Ser/Thr) phosphatase inhibitor okadaic acid, dibutyryl-cAMP-stimulated transcription from the phosphoenolpyruvate carboxykinase (PEPCK) promoter was enhanced over the level of PEPCK gene transcription observed in cells treated with dibutyryl-cAMP alone. This process was mediated, at least in part, by a region of the PEPCK promoter that binds CREB. Likewise, okadaic acid prevents the dephosphorylation of PKA-phosphorylated CREB in rat liver nuclear extracts and enhances the ability of PKA to stimulate transcription from the PEPCK promoter in cell-free reactions. The ability of okadaic acid to enhance PKA-stimulated transcription in vitro was entirely dependent on the presence of CREB in the reactions. The phospho-CREB (P-CREB) phosphatase activity present in nuclear extracts coelutes with protein Ser/Thr phosphatase type 2A (PP2A) on Mono Q, amino-hexyl Sepharose, and heparin agarose columns and was chromatographically resolved from nuclear protein Ser/Thr-phosphatase type 1 (PP1). Furthermore, P-CREB phosphatase activity in nuclear extracts was unaffected by the heat-stable protein inhibitor-2, which is a potent and selective inhibitor of PP1. Nuclear PP2A dephosphorylated P-CREB 30-fold more efficiently than did nuclear PP1. Finally, when PKA-phosphorylated CREB was treated with immunopurified PP2A and PP1, the PP2A-treated CREB did not stimulate transcription from the PEPCK promoter in vitro, whereas the PP1-treated CREB retained the ability to stimulate transcription. Nuclear PP2A appears to be the primary phosphatase that dephosphorylates PKA-phosphorylated CREB.
Full text
PDF


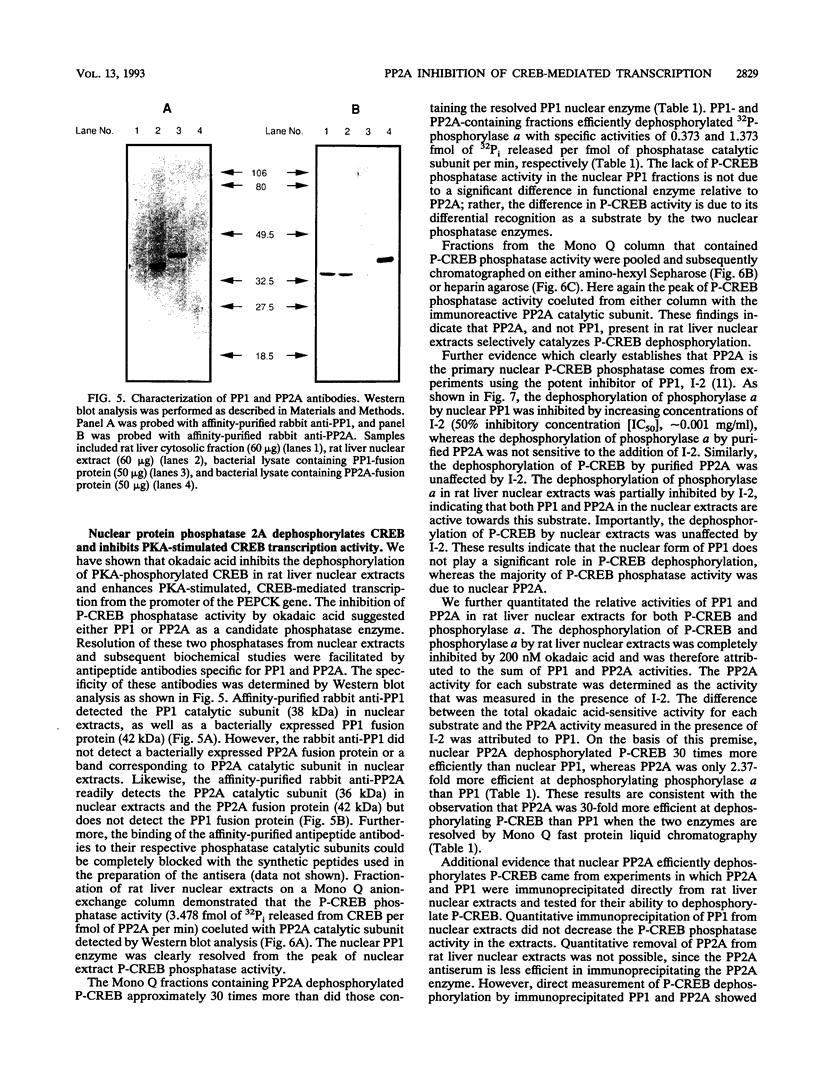
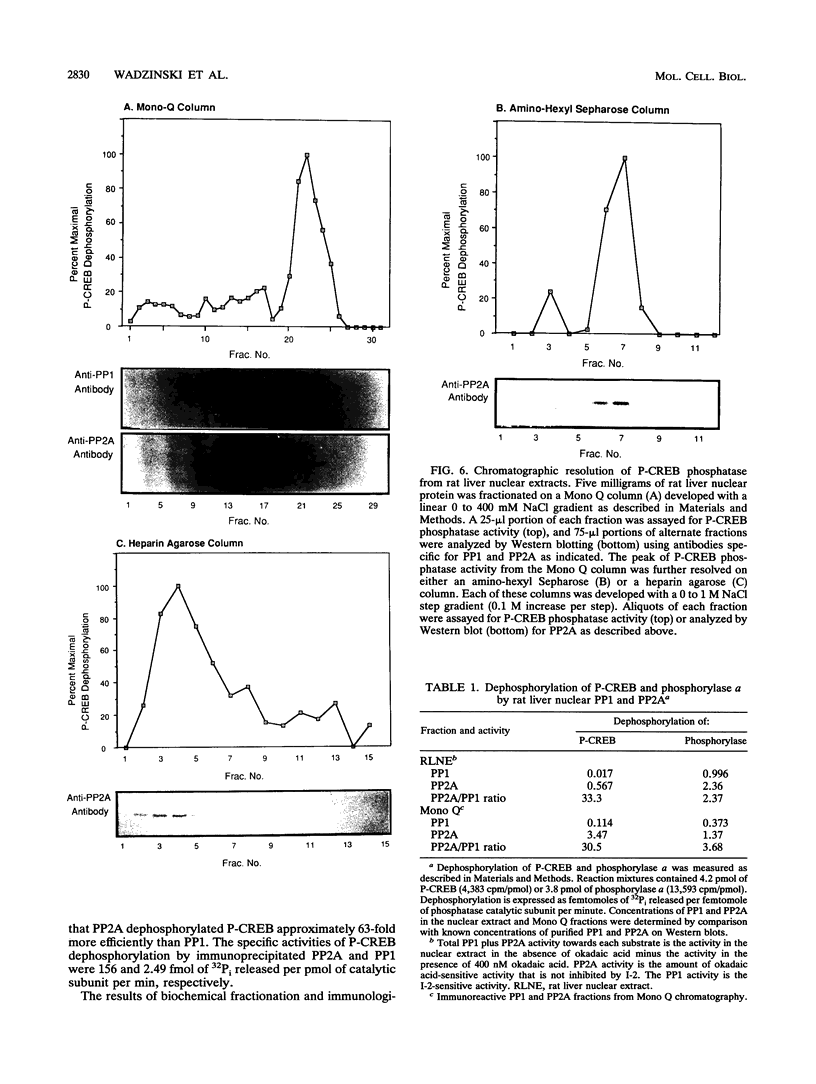
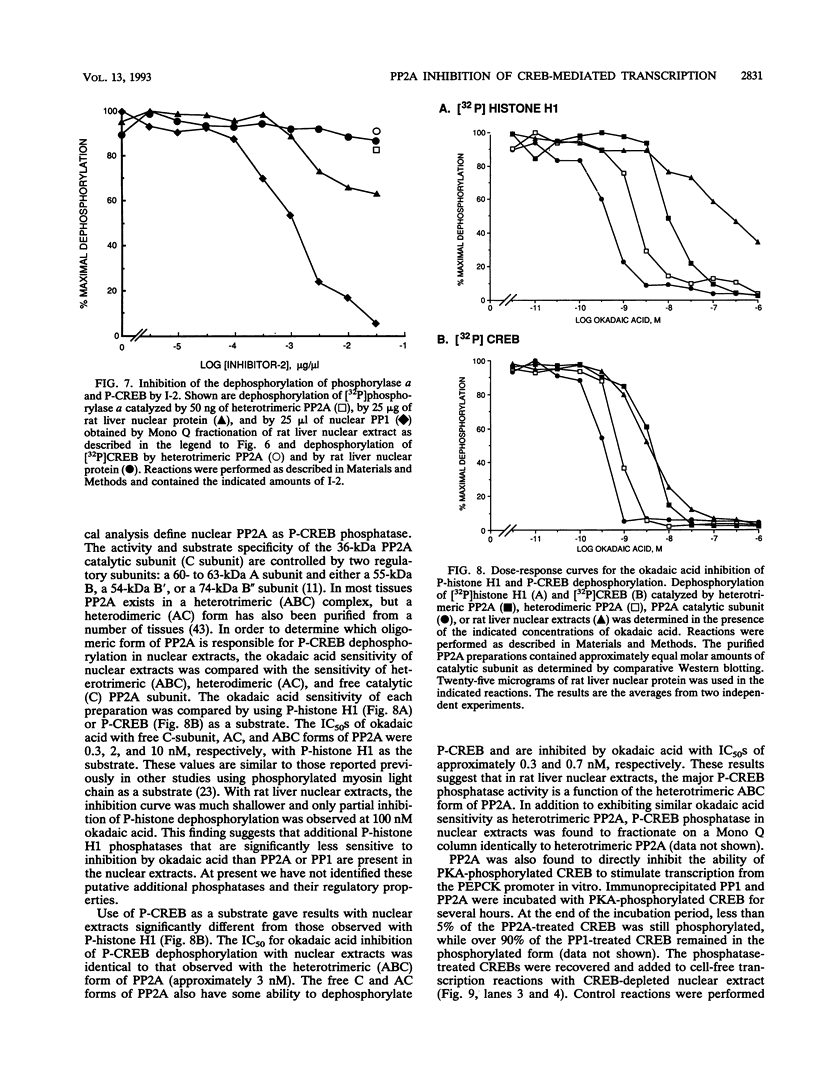
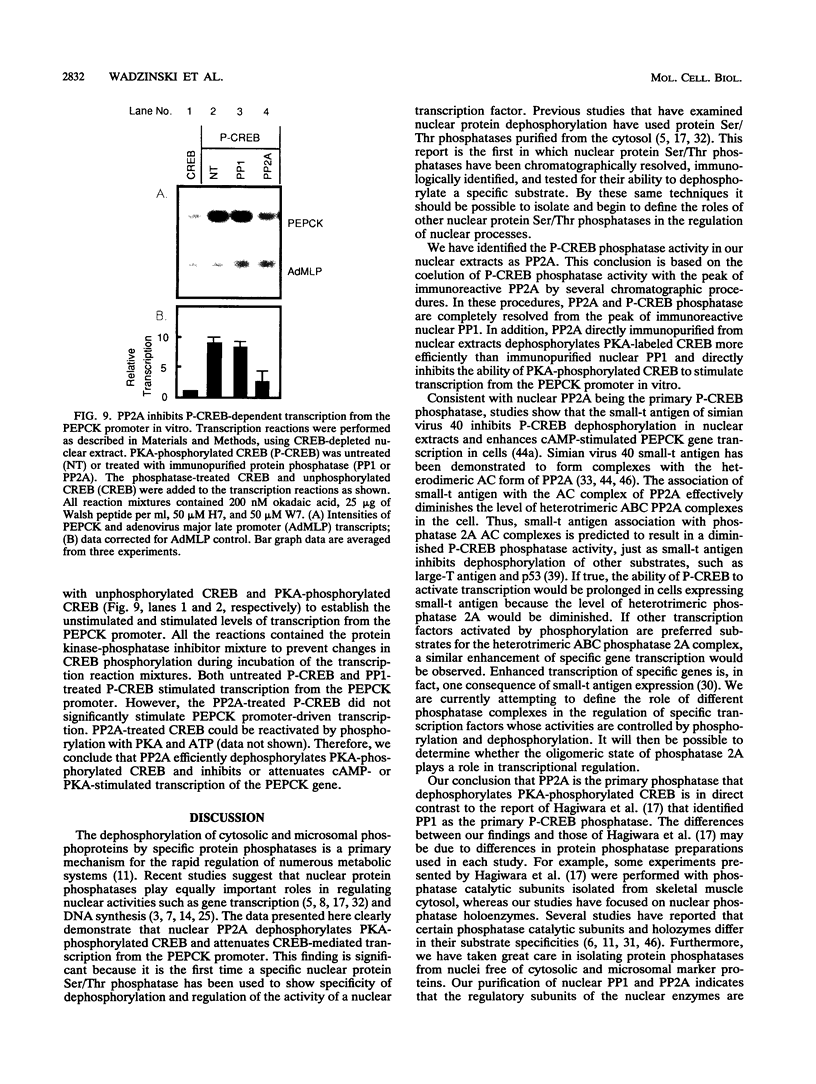


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Antoniw J. F., Nimmo H. G., Yeaman S. J., Cohen P. Comparison of the substrate specificities of protein phosphatases involved in the regulation of glycogen metabolism in rabbit skeletal muscle. Biochem J. 1977 Feb 15;162(2):423–433. doi: 10.1042/bj1620423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arias J. A., Dynan W. S. Promoter-dependent transcription by RNA polymerase II using immobilized enzyme complexes. J Biol Chem. 1989 Feb 25;264(6):3223–3229. [PubMed] [Google Scholar]
- Arndt K. T., Styles C. A., Fink G. R. A suppressor of a HIS4 transcriptional defect encodes a protein with homology to the catalytic subunit of protein phosphatases. Cell. 1989 Feb 24;56(4):527–537. doi: 10.1016/0092-8674(89)90576-x. [DOI] [PubMed] [Google Scholar]
- Bialojan C., Takai A. Inhibitory effect of a marine-sponge toxin, okadaic acid, on protein phosphatases. Specificity and kinetics. Biochem J. 1988 Nov 15;256(1):283–290. doi: 10.1042/bj2560283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black E. J., Street A. J., Gillespie D. A. Protein phosphatase 2A reverses phosphorylation of c-Jun specified by the delta domain in vitro: correlation with oncogenic activation and deregulated transactivation activity of v-Jun. Oncogene. 1991 Nov;6(11):1949–1958. [PubMed] [Google Scholar]
- Bollen M., Stalmans W. The structure, role, and regulation of type 1 protein phosphatases. Crit Rev Biochem Mol Biol. 1992;27(3):227–281. doi: 10.3109/10409239209082564. [DOI] [PubMed] [Google Scholar]
- Booher R., Beach D. Involvement of a type 1 protein phosphatase encoded by bws1+ in fission yeast mitotic control. Cell. 1989 Jun 16;57(6):1009–1016. doi: 10.1016/0092-8674(89)90339-5. [DOI] [PubMed] [Google Scholar]
- Boyle W. J., Smeal T., Defize L. H., Angel P., Woodgett J. R., Karin M., Hunter T. Activation of protein kinase C decreases phosphorylation of c-Jun at sites that negatively regulate its DNA-binding activity. Cell. 1991 Feb 8;64(3):573–584. doi: 10.1016/0092-8674(91)90241-p. [DOI] [PubMed] [Google Scholar]
- Brautigan D. L., Shriner C. L. Methods to distinguish various types of protein phosphatase activity. Methods Enzymol. 1988;159:339–346. doi: 10.1016/0076-6879(88)59034-1. [DOI] [PubMed] [Google Scholar]
- Cheng H. C., Kemp B. E., Pearson R. B., Smith A. J., Misconi L., Van Patten S. M., Walsh D. A. A potent synthetic peptide inhibitor of the cAMP-dependent protein kinase. J Biol Chem. 1986 Jan 25;261(3):989–992. [PubMed] [Google Scholar]
- Cohen P., Klumpp S., Schelling D. L. An improved procedure for identifying and quantitating protein phosphatases in mammalian tissues. FEBS Lett. 1989 Jul 3;250(2):596–600. doi: 10.1016/0014-5793(89)80803-8. [DOI] [PubMed] [Google Scholar]
- Cohen P. The structure and regulation of protein phosphatases. Annu Rev Biochem. 1989;58:453–508. doi: 10.1146/annurev.bi.58.070189.002321. [DOI] [PubMed] [Google Scholar]
- Doonan J. H., Morris N. R. The bimG gene of Aspergillus nidulans, required for completion of anaphase, encodes a homolog of mammalian phosphoprotein phosphatase 1. Cell. 1989 Jun 16;57(6):987–996. doi: 10.1016/0092-8674(89)90337-1. [DOI] [PubMed] [Google Scholar]
- Gonzalez G. A., Montminy M. R. Cyclic AMP stimulates somatostatin gene transcription by phosphorylation of CREB at serine 133. Cell. 1989 Nov 17;59(4):675–680. doi: 10.1016/0092-8674(89)90013-5. [DOI] [PubMed] [Google Scholar]
- Gorski K., Carneiro M., Schibler U. Tissue-specific in vitro transcription from the mouse albumin promoter. Cell. 1986 Dec 5;47(5):767–776. doi: 10.1016/0092-8674(86)90519-2. [DOI] [PubMed] [Google Scholar]
- Hagiwara M., Alberts A., Brindle P., Meinkoth J., Feramisco J., Deng T., Karin M., Shenolikar S., Montminy M. Transcriptional attenuation following cAMP induction requires PP-1-mediated dephosphorylation of CREB. Cell. 1992 Jul 10;70(1):105–113. doi: 10.1016/0092-8674(92)90537-m. [DOI] [PubMed] [Google Scholar]
- Hoeffler J. P., Meyer T. E., Yun Y., Jameson J. L., Habener J. F. Cyclic AMP-responsive DNA-binding protein: structure based on a cloned placental cDNA. Science. 1988 Dec 9;242(4884):1430–1433. doi: 10.1126/science.2974179. [DOI] [PubMed] [Google Scholar]
- Hubbard M. J., Cohen P. Targeting subunits for protein phosphatases. Methods Enzymol. 1991;201:414–427. doi: 10.1016/0076-6879(91)01038-4. [DOI] [PubMed] [Google Scholar]
- Jakes S., Schlender K. K. Histone H1 phosphorylated by protein kinase C is a selective substrate for the assay of protein phosphatase 2A in the presence of phosphatase 1. Biochim Biophys Acta. 1988 Oct 13;967(1):11–16. doi: 10.1016/0304-4165(88)90182-1. [DOI] [PubMed] [Google Scholar]
- Jaspers S. R., Miller T. B., Jr Purification and the immunological characterization of rat protein phosphatase 2A: enzyme levels in diabetic liver and heart. Mol Cell Biochem. 1991 Mar 13;101(2):167–174. doi: 10.1007/BF00229533. [DOI] [PubMed] [Google Scholar]
- Kadonaga J. T., Tjian R. Affinity purification of sequence-specific DNA binding proteins. Proc Natl Acad Sci U S A. 1986 Aug;83(16):5889–5893. doi: 10.1073/pnas.83.16.5889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamibayashi C., Estes R., Slaughter C., Mumby M. C. Subunit interactions control protein phosphatase 2A. Effects of limited proteolysis, N-ethylmaleimide, and heparin on the interaction of the B subunit. J Biol Chem. 1991 Jul 15;266(20):13251–13260. [PubMed] [Google Scholar]
- Kazlauskas A., Cooper J. A. Autophosphorylation of the PDGF receptor in the kinase insert region regulates interactions with cell proteins. Cell. 1989 Sep 22;58(6):1121–1133. doi: 10.1016/0092-8674(89)90510-2. [DOI] [PubMed] [Google Scholar]
- Kinoshita N., Ohkura H., Yanagida M. Distinct, essential roles of type 1 and 2A protein phosphatases in the control of the fission yeast cell division cycle. Cell. 1990 Oct 19;63(2):405–415. doi: 10.1016/0092-8674(90)90173-c. [DOI] [PubMed] [Google Scholar]
- Kris R. M., Lax I., Gullick W., Waterfield M. D., Ullrich A., Fridkin M., Schlessinger J. Antibodies against a synthetic peptide as a probe for the kinase activity of the avian EGF receptor and v-erbB protein. Cell. 1985 Mar;40(3):619–625. doi: 10.1016/0092-8674(85)90210-7. [DOI] [PubMed] [Google Scholar]
- Lee C. Q., Yun Y. D., Hoeffler J. P., Habener J. F. Cyclic-AMP-responsive transcriptional activation of CREB-327 involves interdependent phosphorylated subdomains. EMBO J. 1990 Dec;9(13):4455–4465. doi: 10.1002/j.1460-2075.1990.tb07896.x. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Lewis E. J., Harrington C. A., Chikaraishi D. M. Transcriptional regulation of the tyrosine hydroxylase gene by glucocorticoid and cyclic AMP. Proc Natl Acad Sci U S A. 1987 Jun;84(11):3550–3554. doi: 10.1073/pnas.84.11.3550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loeken M., Bikel I., Livingston D. M., Brady J. trans-activation of RNA polymerase II and III promoters by SV40 small t antigen. Cell. 1988 Dec 23;55(6):1171–1177. doi: 10.1016/0092-8674(88)90261-9. [DOI] [PubMed] [Google Scholar]
- Mumby M. C., Russell K. L., Garrard L. J., Green D. D. Cardiac contractile protein phosphatases. Purification of two enzyme forms and their characterization with subunit-specific antibodies. J Biol Chem. 1987 May 5;262(13):6257–6265. [PubMed] [Google Scholar]
- Nichols M., Weih F., Schmid W., DeVack C., Kowenz-Leutz E., Luckow B., Boshart M., Schütz G. Phosphorylation of CREB affects its binding to high and low affinity sites: implications for cAMP induced gene transcription. EMBO J. 1992 Sep;11(9):3337–3346. doi: 10.1002/j.1460-2075.1992.tb05412.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pallas D. C., Shahrik L. K., Martin B. L., Jaspers S., Miller T. B., Brautigan D. L., Roberts T. M. Polyoma small and middle T antigens and SV40 small t antigen form stable complexes with protein phosphatase 2A. Cell. 1990 Jan 12;60(1):167–176. doi: 10.1016/0092-8674(90)90726-u. [DOI] [PubMed] [Google Scholar]
- Pallas D. C., Weller W., Jaspers S., Miller T. B., Lane W. S., Roberts T. M. The third subunit of protein phosphatase 2A (PP2A), a 55-kilodalton protein which is apparently substituted for by T antigens in complexes with the 36- and 63-kilodalton PP2A subunits, bears little resemblance to T antigens. J Virol. 1992 Feb;66(2):886–893. doi: 10.1128/jvi.66.2.886-893.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park E. A., Roesler W. J., Liu J., Klemm D. J., Gurney A. L., Thatcher J. D., Shuman J., Friedman A., Hanson R. W. The role of the CCAAT/enhancer-binding protein in the transcriptional regulation of the gene for phosphoenolpyruvate carboxykinase (GTP). Mol Cell Biol. 1990 Dec;10(12):6264–6272. doi: 10.1128/mcb.10.12.6264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinn P. G., Granner D. K. Cyclic AMP-dependent protein kinase regulates transcription of the phosphoenolpyruvate carboxykinase gene but not binding of nuclear factors to the cyclic AMP regulatory element. Mol Cell Biol. 1990 Jul;10(7):3357–3364. doi: 10.1128/mcb.10.7.3357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roesler W. J., Vandenbark G. R., Hanson R. W. Identification of multiple protein binding domains in the promoter-regulatory region of the phosphoenolpyruvate carboxykinase (GTP) gene. J Biol Chem. 1989 Jun 5;264(16):9657–9664. [PubMed] [Google Scholar]
- Sasaki K., Cripe T. P., Koch S. R., Andreone T. L., Petersen D. D., Beale E. G., Granner D. K. Multihormonal regulation of phosphoenolpyruvate carboxykinase gene transcription. The dominant role of insulin. J Biol Chem. 1984 Dec 25;259(24):15242–15251. [PubMed] [Google Scholar]
- Scheidtmann K. H., Mumby M. C., Rundell K., Walter G. Dephosphorylation of simian virus 40 large-T antigen and p53 protein by protein phosphatase 2A: inhibition by small-t antigen. Mol Cell Biol. 1991 Apr;11(4):1996–2003. doi: 10.1128/mcb.11.4.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shenolikar S., Ingebritsen T. S. Protein (serine and threonine) phosphate phosphatases. Methods Enzymol. 1984;107:102–129. doi: 10.1016/0076-6879(84)07007-5. [DOI] [PubMed] [Google Scholar]
- Short J. M., Wynshaw-Boris A., Short H. P., Hanson R. W. Characterization of the phosphoenolpyruvate carboxykinase (GTP) promoter-regulatory region. II. Identification of cAMP and glucocorticoid regulatory domains. J Biol Chem. 1986 Jul 25;261(21):9721–9726. [PubMed] [Google Scholar]
- Studier F. W., Rosenberg A. H., Dunn J. J., Dubendorff J. W. Use of T7 RNA polymerase to direct expression of cloned genes. Methods Enzymol. 1990;185:60–89. doi: 10.1016/0076-6879(90)85008-c. [DOI] [PubMed] [Google Scholar]
- Usui H., Imazu M., Maeta K., Tsukamoto H., Azuma K., Takeda M. Three distinct forms of type 2A protein phosphatase in human erythrocyte cytosol. J Biol Chem. 1988 Mar 15;263(8):3752–3761. [PubMed] [Google Scholar]
- Walter G., Ruediger R., Slaughter C., Mumby M. Association of protein phosphatase 2A with polyoma virus medium tumor antigen. Proc Natl Acad Sci U S A. 1990 Apr;87(7):2521–2525. doi: 10.1073/pnas.87.7.2521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto K. K., Gonzalez G. A., Biggs W. H., 3rd, Montminy M. R. Phosphorylation-induced binding and transcriptional efficacy of nuclear factor CREB. Nature. 1988 Aug 11;334(6182):494–498. doi: 10.1038/334494a0. [DOI] [PubMed] [Google Scholar]
- Yang S. D., Vandenheede J. R., Merlevede W. A simplified procedure for the purification of the protein phosphatase modulator (inhibitor-2) from rabbit skeletal muscle. FEBS Lett. 1981 Sep 28;132(2):293–295. doi: 10.1016/0014-5793(81)81182-9. [DOI] [PubMed] [Google Scholar]
- Yang S. I., Lickteig R. L., Estes R., Rundell K., Walter G., Mumby M. C. Control of protein phosphatase 2A by simian virus 40 small-t antigen. Mol Cell Biol. 1991 Apr;11(4):1988–1995. doi: 10.1128/mcb.11.4.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Wet J. R., Wood K. V., DeLuca M., Helinski D. R., Subramani S. Firefly luciferase gene: structure and expression in mammalian cells. Mol Cell Biol. 1987 Feb;7(2):725–737. doi: 10.1128/mcb.7.2.725. [DOI] [PMC free article] [PubMed] [Google Scholar]