Figure 1. MMOB induces conformational changes that affect function.
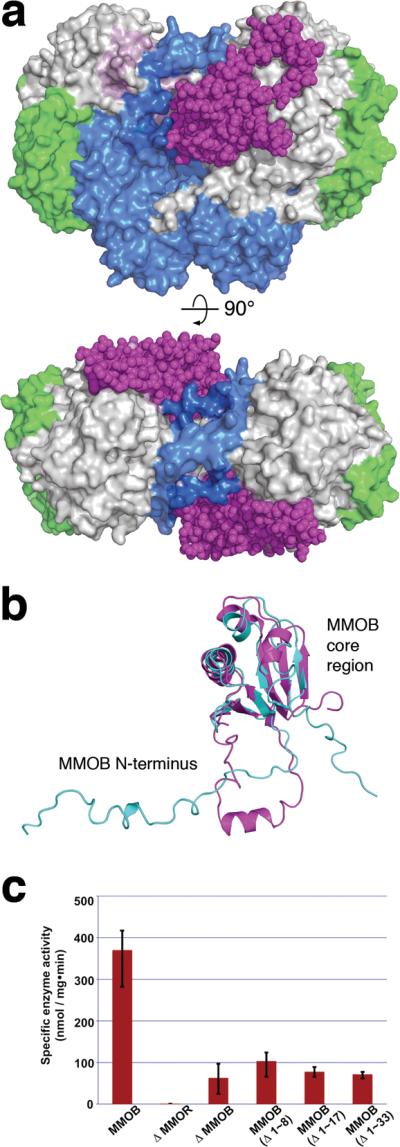
(a) Front (upper) and top (lower) views of a space-filling representation of the H-B complex. MMOB (magenta) binds to the canyon region formed by the α- (grey) and β- (blue) subunits of MMOH. The MMOH γ-subunit is depicted in green. (b) Structural alignment of the solution NMR structure of MMOB from M. capsulatus (Bath) (PDB Code: 1CKV) (cyan) with that of MMOB in the H-B complex (magenta). An α helix (Gly 17-Phe 25) forms in the MMOB N-terminus upon complexation with MMOH. (c) sMMO activity assay with wild type and truncated versions of MMOB. Propylene is converted to propylene oxide in the presence of NADH. Native MMOB is required for maximum sMMO activity. N-terminal truncated MMOB constructs tested, (Δ1–8), (Δ1–17), and (Δ1–33) show activity profiles similar to that observed in the absence of MMOB (n = 3, mean values ± deviation).
