Figure 4. Coordination geometry at the diiron active site of MMOH.
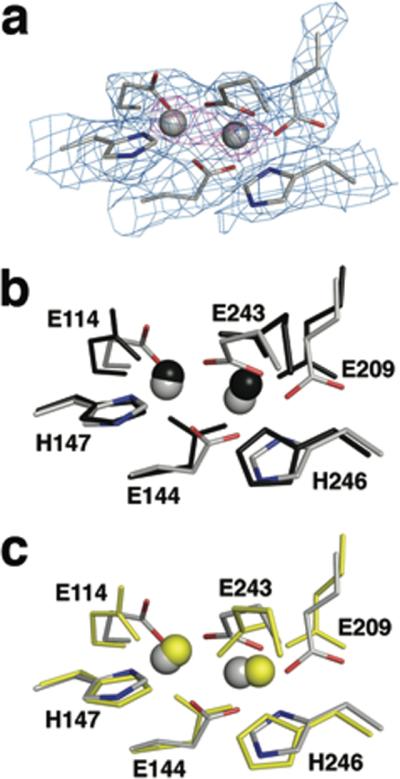
(a) Electron density at the diiron site in H-B. Side chain ligands are shown as sticks in grey (carbon), blue (nitrogen), and red (oxygen). 2Fo-Fc electron density at 1.0 sigma and 5.0 sigma is drawn as a mesh in light blue and magenta, respectively. Views comparing the diiron site in H-B (grey) with that in MMOHox (b, PDB code: 1MTY, black) and in MMOHred (c, PDB code: 1FYZ, yellow) are also presented in (b) and (c), respectively. Upon MMOB binding, Glu 243 undergoes a substantial conformational change, involving simultaneous chelation of Fe2 and bridging to Fe1. Such bidentate coordination of the Glu 243 side chain resembles that in MMOHred, but distances between the carboxylate oxygen atoms and Fe2 (OE1-Fe2 and OE2-Fe2) are shorter in H-B (1.9 and 2.0 Å) than in MMOHred (2.4 and 2.4 Å). This result is more consistent with an Fe(III) than an Fe(II) oxidation state for the iron atoms in H-B. Solvent-derived ligands, such as hydroxide ion and water, are not observed in the H-B complex owing to the 2.9 Å resolution.
