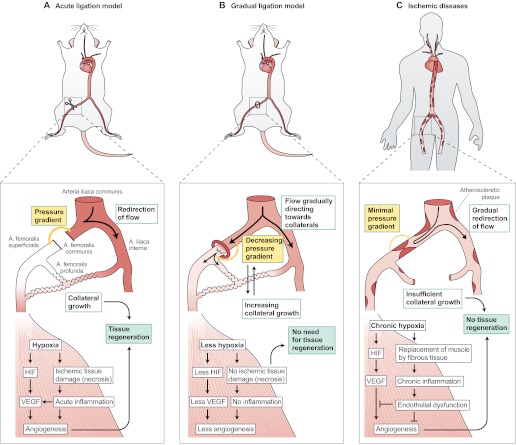
Fig. 2.
Differences in collateral growth and tissue recovery in animal models versus humans with arterial occlusive disease. (A) After an acute arterial ligation (shown here in a mouse), there is a strong pressure gradient between the proximal and distal sides of the occlusion (orange line). This redirects the blood flow into adjacent arterioles and causes a strong shear-stress-mediated opening of collateral channels, which restore blood flow into the hypoxic areas. In the hypoxic tissues, ischemic tissue damage (necrosis) occur if the blood flow is not restored within the first hours after the occlusion. Necrosis induces acute inflammation, and recruited inflammatory cells produce angiogenic cytokines such as VEGF. The hypoxia itself activates factors such as HIF that stimulate the production of VEGF among other factors, and angiogenesis. Distal angiogenesis, along with the growth of collaterals, contributes to the tissue recovery by the formation of connections between the collaterals and the distal capillaries. (B) Upon a gradual occlusion, for example by using an ameroid constrictor, the slow development of the occlusion produces an accommodation window for the collaterals to open. The pressure gradient is never as high as after acute ligation because the blood flow is gradually redirected to the adjacent arterioles, which have time to open to balance the pressure change caused by the gradual occlusion with the constrictor. There is no acute tissue damage, because the collaterals start to compensate for the lack of blood flow before it completely stops. Therefore, there is also less inflammation. The amount of hypoxia and tissue damage varies according to the availability of collaterals and causes comparable distal angiogenic responses. If collaterals are effectively recruited, there is very little tissue hypoxia and therefore little endogenous growth factor upregulation, but also no tissue damage requiring recovery. (C) In human ischemic diseases, atherosclerotic plaques develop all around the arterial tree, causing blood pressure to decrease gradually following each plaque. Thus, a single occlusion in the periphery causes a very small pressure gradient between the proximal and distal sides of the occlusion, as the blood pressure is low already above the occlusion. Accordingly, there is very little blood flow to redirect towards the collaterals and minimal shear stress to open the collaterals, which results in insufficient collateralization. Distal tissue suffers from gradually developing chronic hypoxia that manifests by the replacement of muscle fibers with less-energy-consuming fibrotic tissue and chronic inflammation. The tissue might be less prone to respond to angiogenic stimuli owing to endothelial dysfunction and other factors. The insufficient collaterals and angiogenic signaling limit tissue recovery, eventually leading to total necrosis and loss of tissue function if not effectively treated. Artery names are shown in panel A.