FIG. 3. Tyrosine 219 phosphorylation on MKK6 is important for binding to Rac1.
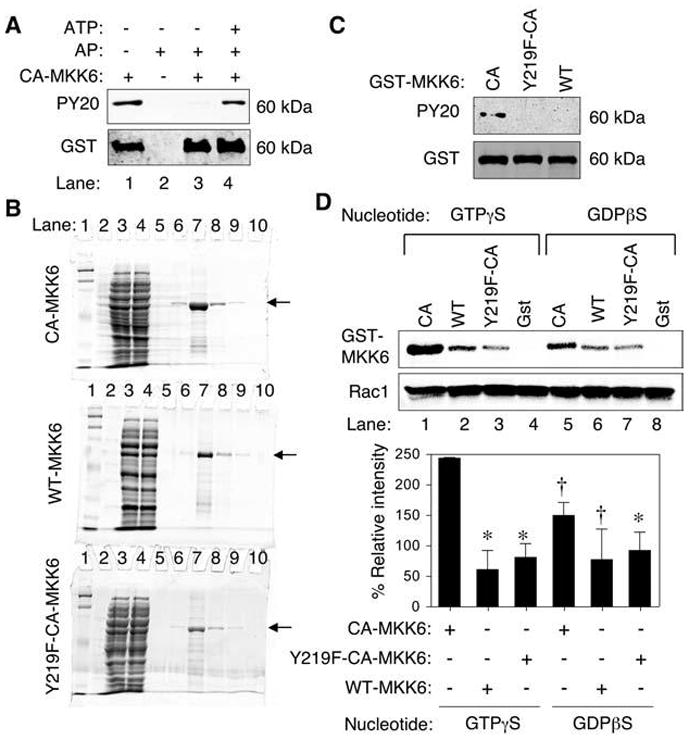
(A) Phosphotyrosine (PY20) Western blot analysis of purified GST-CA-MKK6 demonstrating that bacteria expressed CA-MKK6 is tyrosine phosphorylated (lane 1). Western blots were probed with anti-phosphotyrosine antibody (PY20) or anti-GST as a loading control. Purified GST-CA-MKK6 was treated with alkaline phosphatase (AP) before Western blotting (lane 3) and then reincubated with ATP after removal of alkaline phosphatase (lane 4). (B) Purity of GST-tagged MKK6 proteins. Coomassie-stained SDS-PAGE for GST-MKK6–purified proteins (arrow). Lanes are as follows: 1 and 2 (MW markers), 3 (crude bacterial lysate), 4 (GST-column flow through), 5–10 (purification fractions with lane 7 containing the peak protein fraction). (C) Western blot of MKK6 fusion proteins probed with PY20 and anti-GST antibodies. Both the GST-Y219F-CA-MKK6 mutant and GST-WT-MKK6 failed to demonstrate tyrosine phosphorylation in comparison to GST-CA-MKK6. (D) In vitro pull-down of purified His-tagged Rac1 in the presence of purified constitutively active (CA), wild-type (WT), tyrosine mutant (Y219F-CA) GST-MKK6 proteins followed by Western blotting for GST and Rac1 (upper panel). Free GST protein was also used as a negative control in these studies. The His-tagged Rac1 was preloaded with the indicated nucleotide analogues before incubation with MKK6 or GST proteins. Lower panel, quantification of GST-MKK6 bands relative to Rac1 bands from three independent experiments. The relative intensity was normalized for each experiment to values obtained from CA-MKK6 (GTP) before calculating the mean ± SEM for the three experiments. Marked values are significantly different from the CA-MKK6 + GTPγS-Rac1 group; *p < 0.01; †p < 0.05; Student’s t test.
