Figure 3. Dynamin-dependent endocytosis is required for the redistribution of c-Src following H/R injury.
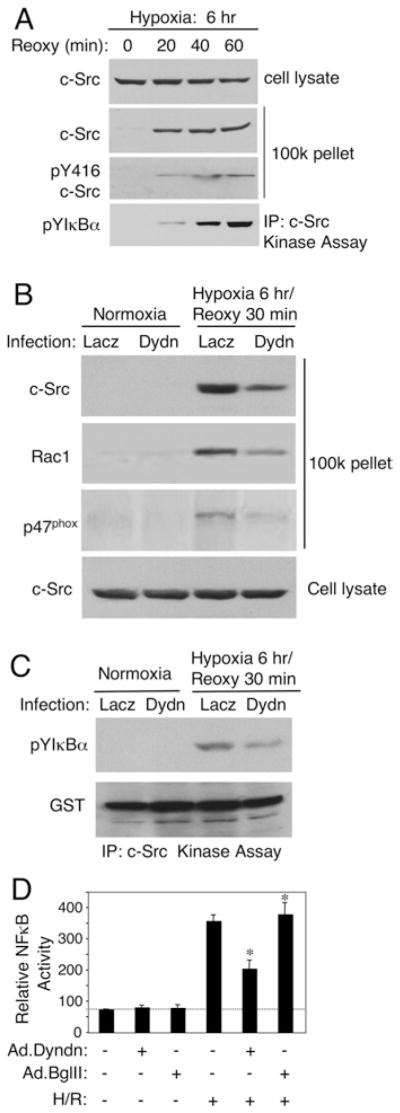
(A) HeLa cells were exposed to 6 h of hypoxia and the indicated periods of reoxygenation (Reoxy) prior to cell harvesting. A 1 μg portion of each endomembrane sample (100k pellet) was used to evaluate both total c-Src recruitment to endomembranes and c-Src activation (using an anti-pY416-c-Src antibody). In addition, c-Src was immunoprecipitated from 500 μg of total cell lysate, and its ability to tyrosine-phosphorylate GST–IκBα was then evaluated using a non-radioactive in vitro kinase assay. Tyrosine phosphorylation of IκBα (pYIκBα) was evaluated by Western blotting with an anti-phosphotyrosine (PY20) antibody. (B) HeLa cells were infected with Ad.DynK44A (Dydn, a dominant-negative mutant of dynamin) or Ad.LacZ (viral infection control) 48 h prior to H/R injury (by 6 h hypoxia and 30 min reoxygenation). Endomembrane recruitment of the indicated proteins was examined by Western blotting. (C) HeLa cells were infected with Ad.DynK44A or Ad.Lacz 48 h prior to H/R injury (6 h hypoxia and 30 min reoxygenation). c-Src was immunoprecipitated and its ability to tyrosine-phosphorylate GST–IκBα directly was evaluated using a non-radioactive in vitro kinase assay followed by Western blotting with anti-phosphotyrosine (PY20) antibody. Anti-GST antibody was used as a loading control. (D) HeLa cells were infected with Ad.DynK44A or Ad.BglII 48 h prior to H/R injury, and with Ad.NF-κBLuc 24 h prior to H/R injury. Luciferase activities were assessed following 6 h of hypoxia and 6 h of reoxygenation (mean ± S.E.M., n = 3). The ‘*’ symbol indicates a statistically significant difference using ANOVA followed by the Dunnett’s post-hoc test (P < 0.01). IP, immunoprecipitaion.
