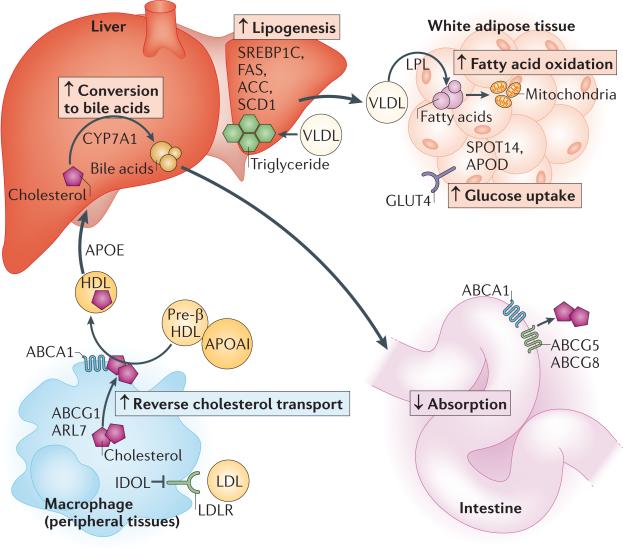
Figure 2. Coordinated effects of LXR on metabolism.
Liver X receptor (LXR) mediates effects on multiple metabolic pathways in a tissue-specific manner. In peripheral cells such as macrophages, LXR induces expression of IDOL (inducible degrader of the low-density lipoprotein receptor (LDLR)), which promotes the proteasome-mediated degradation of LDLR and thus results in reduced LDL uptake into the cell. In peripheral cells, LXR also increases ARL7 (ADP-ribosylation factor-like 7), ABCA1 (ATP-binding cassette transporter A1) and ABCG1 expression, promoting the movement of cholesterol to the plasma membrane and cholesterol efflux and transfer to lipid-poor molecules such as apolipoprotein AI (APOAI) and pre-β high-density lipoprotein (HDL), thus increasing plasma HDL levels. APOE promotes the return of HDL to the liver. Furthermore, in the liver, LXR promotes cholesterol conversion to bile acids by cytochrome P450 7A1 (CYP7A1). It also promotes fatty acid synthesis via induction of sterol-regulatory element-binding protein 1C (SREBP1C) and its targets, fatty acid synthase (FAS), acetyl CoA carboxylase (ACC) and steroyl CoA desaturase 1 (SCD1). Secretion of triglyceride-rich very low-density lipoproteins (VLDLs) by the liver transports lipids to peripheral tissues, including adipose tissue, where the action of lipoprotein lipase (LPL) liberates fatty acids from VLDL. In adipose tissue, LXR regulates the expression of lipid-binding and metabolic proteins such as APOD and SPOT14 and may promote the breakdown of fatty acids through their β-oxidation (which occurs in mitochondria). LXR also promotes glucose uptake via induction of glucose transporter type 4 (GLUT4). Finally, in the intestine, LXR inhibits cholesterol absorption by inducing the expression of the ABC transporters ABCG5 and ABCG8 and possibly ABCA1.