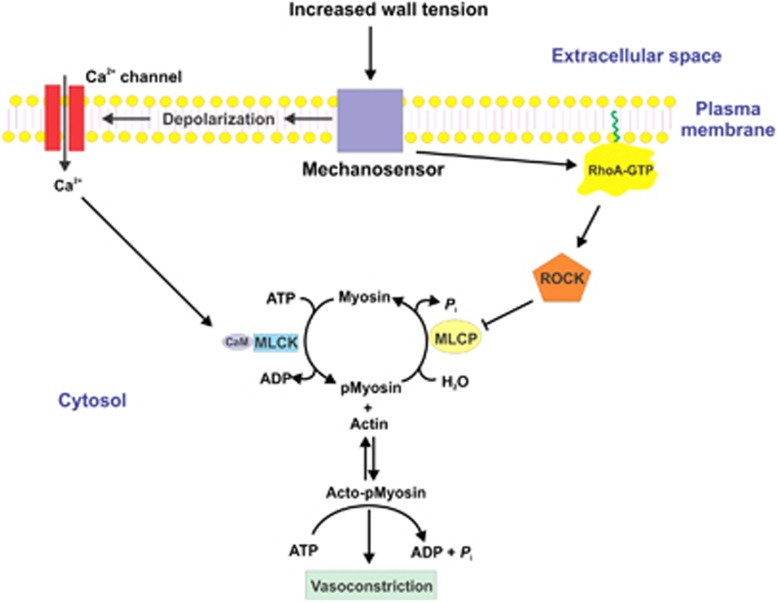
Figure 3.
Pressure-induced vasoconstriction via increased [Ca2+]i and via Ca2+ sensitization mediated by inhibition of myosin light chain phosphatase (MLCP). The mechanosensor detects an increase in arterial wall tension, which leads to membrane depolarization, opening of voltage-gated Ca2+ channels, and influx of Ca2+ from the extracellular space. Extracellular-free [Ca2+] is ∼2.5 mM and cytoplasmic-free [Ca2+] at rest is ∼120 to 150 nmol/L. After stimulation, the free [Ca2+] in the cytoplasm rises to ∼300 to 500 nmol/L. This increase in [Ca2+]i saturates the four Ca2+-binding sites of calmodulin (CaM), leading to activation of Ca2+/CaM-dependent myosin light chain kinase (MLCK). Activated MLCK phosphorylates the two 20-kDa light chain subunits of myosin II at Ser-19, which activates cross-bridge cycling and vasoconstriction, driven by the energy derived from the hydrolysis of ATP within the head regions of phosphorylated myosin bound to actin filaments. Detection of an increase in wall tension by the mechanosensor also leads to activation of the small GTPase RhoA. The mechanism of activation of RhoA likely involves activation of a guanine nucleotide exchange factor, which catalyzes GDP-GTP exchange on RhoA. This induces dissociation of a guanine nucleotide dissociation inhibitor and a conformational change involving exposure of a hydrophobic geranylgeranyl moiety on RhoA. RhoA-GTP then translocates from the cytosol to the plasma membrane where it attaches to the membrane via the geranylgeranyl moiety (indicated in green) and activates Rho-associated kinase (ROCK). Activated ROCK phosphorylates the myosin targeting subunit of MLCP, MYPT1, at Thr-855 (and perhaps Thr-697), resulting in inhibition of phosphatase activity, increased phosphorylation of LC20 at Ser-19 by MLCK, and enhanced contraction, without an additional change in [Ca2+]i. ROCK can also phosphorylate CPI-17 at Thr-18, which thereby becomes a potent inhibitor of MLCP; however, this does not occur during the myogenic response of rat cerebral arteries. Protein kinase C (PKC) can also phosphorylate CPI-17 at Thr-38 but, since this does not occur during the myogenic response of rat cerebral arteries, this mechanism cannot explain the role of PKC in the myogenic response.