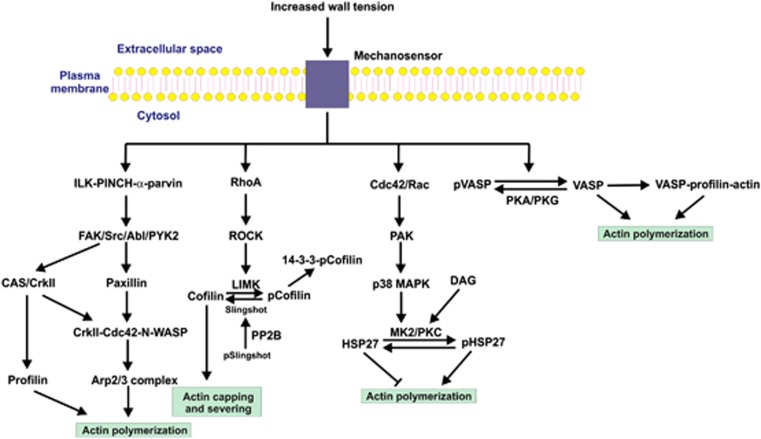
Figure 4.
Potential pathways leading to actin polymerization in the myogenic response. Several signal transduction pathways have been implicated in the regulation of actin polymerization, based largely on studies of agonist stimulation of airway and vascular smooth muscles, but the possibility that one or more of them may have a role in the myogenic response deserves investigation. An early event after stimulation is the recruitment to the plasma membrane of a trimeric complex composed of integrin-linked kinase (ILK), PINCH (particularly interesting new cysteine-histidine-rich protein), and α-parvin. A variety of tyrosine kinases are capable of phosphorylating paxillin, leading to the sequential activation of N-WASP (neuronal Wiskott-Aldrich syndrome protein) and the actin-related protein (Arp)2/3 complex, which activates actin polymerization. N-WASP can also be activated via p130 CAS (Crk-associated substrate) and the adapter protein, CrkII. In addition, the actin-binding protein profilin lies downstream of CAS and induces actin polymerization. In addition to their role in Ca2+ sensitization via myosin light chain phosphatase (MLCP) inhibition, RhoA and Rho-associated kinase (ROCK) may be involved in regulation of actin polymerization through the actin capping and severing protein cofilin. Lin 11, Isl-1, Mec-3 kinase (LIMK) is a substrate of ROCK which, when phosphorylated within its activation loop, phosphorylates cofilin at Ser-3. Phosphocofilin (pCofilin) loses the ability to cap and sever actin filaments, so activation of this pathway would stabilize preformed actin filaments in the subplasmalemmal domain. HSP27 is an actin capping protein that inhibits actin polymerization. This effect is alleviated by phosphorylation (pHSP27) by MK2, which lies downstream of p38 MAPK (mitogen-activated protein kinase) and the small GTPase-activated PAK (p21-activated protein kinase). Protein kinase C (PKC), activated by diacylglycerol (DAG), phosphorylates the same sites in HSP27 as does MK2. The vasodilator-stimulated phosphoprotein (VASP) is a PKA and PKG substrate, with phosphorylation (pVASP) preventing its interaction with actin and the profilin–actin complex. Dephosphorylation favors binding of VASP to G-actin and profilin–actin, which enhance actin polymerization. While the role of VASP has not been studied in relation to the myogenic response, the possibility that increased wall tension leads to dephosphorylation of VASP deserves attention.