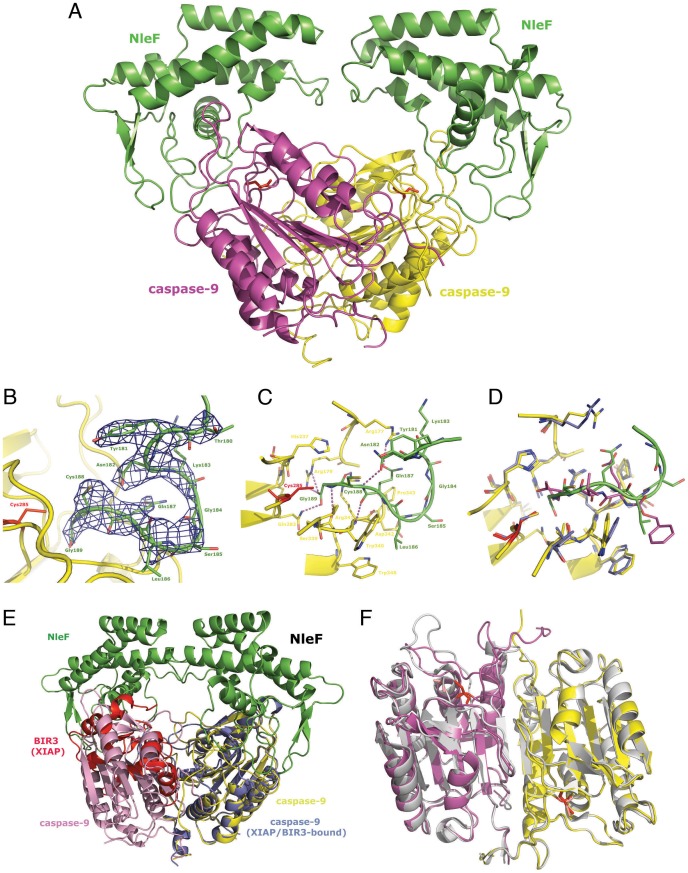
Figure 4. Crystal structure of Caspase-9 with bound NleF.
A. The caspase-9 protease monomers are shown in magenta and yellow, the active site cysteines (Cys285) are shown as red sticks for improved special orientation of the catalytic site. The NleF effector proteins attached to the caspase active sites are shown in green, as labeled in the figure. B. The C-terminal residues of NleF are anchoring the effector protein to the specificity pockets of the protease. The Fo-Fc omit electron density contoured at 2.5 σ is shown in blue for a representative part of the involved C-terminal NleF residues. C. Detailed binding mode of the NleF C-terminus to the caspase-9 active site. Involved amino acids are shown as green and yellow sticks, hydrogen bonds are depicted as magenta dashed lines. D. Superposition of the NleF(green)-bound caspase-9 (yellow) with the peptide inhibitor Z-EVD-Dcbmk (magenta) bound to the caspase-9 monomer (blue) captured in active state (PDB entry 1JXQ). While the conformation of caspase residues is virtually identical, slight differences in the mode of interaction of the bound inhibitors are e.g. observed for the Asp/Gly-P1 moiety, where the Asp of Z-EVD-Dcbmk adopts a more favourable geometry for efficient salt bridge formation to Arg179. E. Superimposition of the NleF-bound caspase-9 dimer presented in this study and the previously described caspase-9 crystal structure (light blue) in complex with the BIR3 domain of the XIAP inhibitor shown as red cartoon (PDB entry 1NW9). While the latter interferes with the formation of a productive caspase dimer, NleF establishes its inhibitory activity by means of a fundamentally different mechanism of active site targeting. F. Superimposition of the caspase-9 dimer obtained for the NleF-inhibited form (yellow and magenta as in A., both NleF molecules omitted for clarity) and the dimer of 1JXQ (grey cartoon). While the type of dimer formation and the overall protein conformation is well conserved between the two dimers, deviations arise mainly from the fact that one of the two 1JXQ monomers (left) corresponds to a non-productive, inactive conformation while in the NleF-inhibited dimer, both monomers adopt an active state with well-formed specificity pockets.