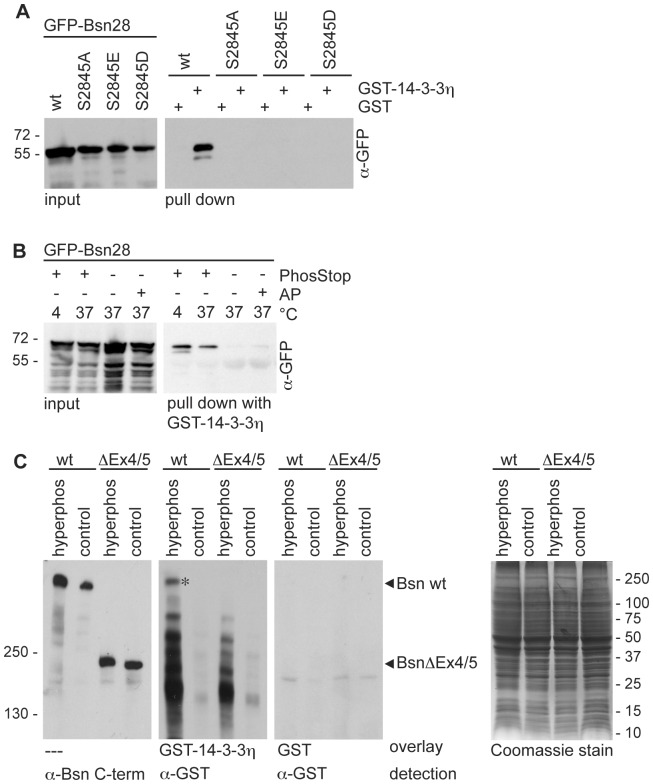
Figure 2. Direct binding of Bassoon to 14-3-3 depends on phosphorylation of Serine-2845. (A).
GFP-Bsn28 wild-type (wt) and its variants containing mutation in the critical S2845 residue (S2845A, S2845E, S2845D) were expressed in HEK293T cells and employed for pull-down experiments with bacterially expressed and purified GST-14-3-3η or GST as a control. Detection of GFP-tagged proteins in the cell lysates (input) and the bound fractions (pull down) was performed using α–GFP antibody. GFP-Bsn28 was successfully co-precipitated by GST-14-3-3η, but not with GST. All tested mutations interfered with the binding. (B) Cell lysates from HEK293T cells expressing GFP-Bsn28 without any additives or supplemented with phosphatase inhibitors (PhosStop) or alkaline phosphatase (AP) and incubated at 4° or 37° C were used for pull-down experiments with immobilized GST-14-3-3η. GFP-Bsn28 was detected in cell lysates (input) and the bound fractions (pull down) using α-GFP antibodies. (C) Hyperphosphorylated and control P2 fractions from brains of wild-type (wt) and Bassoon mutant mice (BsnΔEx4/5) were separated by SDS-PAGE. Equal amounts of protein in each sample was controlled by Coomassie Blue staining for all proteins (right panel). Immunodetection with α-Bsn C-term antibodies revealed the immunoreactivity of wild-type Bassoon (420 kD) and the mutant BassoonΔEx4/5 residual protein (180 kD). Purified GST-14-3-3η or GST fusion proteins were used for the overlay and detected by α-GST antibody. Note the presence of the band (marked by asterisk) corresponding to Bassoon in lysates from wt but not from BsnΔEx4/5 mice showing binding GST-14-3-3h, but not GST fusion protein. Bars and number on the left side of the blots and on the right side of Coomassie-stained gel show sizes and positions of molecular weight markers. Images shown in this panel are representative for results obtained in at least two independent experiments