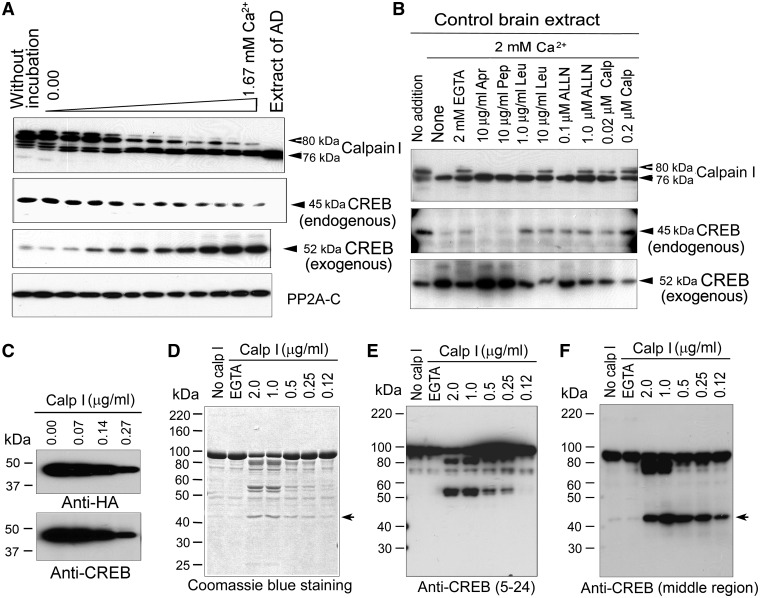
Figure 6.
Proteolysis of CREB is catalyzed by calcium-mediated truncation/activation of calpain I. (A) In vitro proteolyses of calpain I and CREB in human brain extracts were activated by calcium. A normal human brain extract was incubated for 10 min at 30°C in the absence (upper two panels) or presence (3th panel) of recombinant MBP–CREB and analyzed by western blots. PP2A catalytic subunit was used as negative control. (B) Calcium-activated proteolysis of calpain I and CREB was inhibited by calpain inhibitors selectively. Recombinant MBP–CREB was incubated with normal human brain extract in the presence of EGTA or CaCl2 plus various selective protease inhibitors for 10 min at 30°C, followed by western blots to detect the proteolysis. Abbreviations: Apr, aprotinin; Pep, pepstatin; Leu, leupeptin; Calp, calpastatin peptide. (C) Immunopurified CREB from mammalian cells was proteolysed by calpain I. HA-CREB was immunopurified from HEK-293FT cells and incubated with various concentrations of calpain I for 10 min at 30°C. The reaction products were analyzed with western blots. (D–F) Recombinant MBP–CREB was proteolysed by calpain I. Recombinant MBP–CREB was incubated with various concentrations of calpain I for 10 min at 30°C. The proteolytic products were analyzed by Coomassie blue staining (D) and western blots developed with anti-CREB (against amino acids 5–24) (E) or anti-CREB (against middle region) (F).