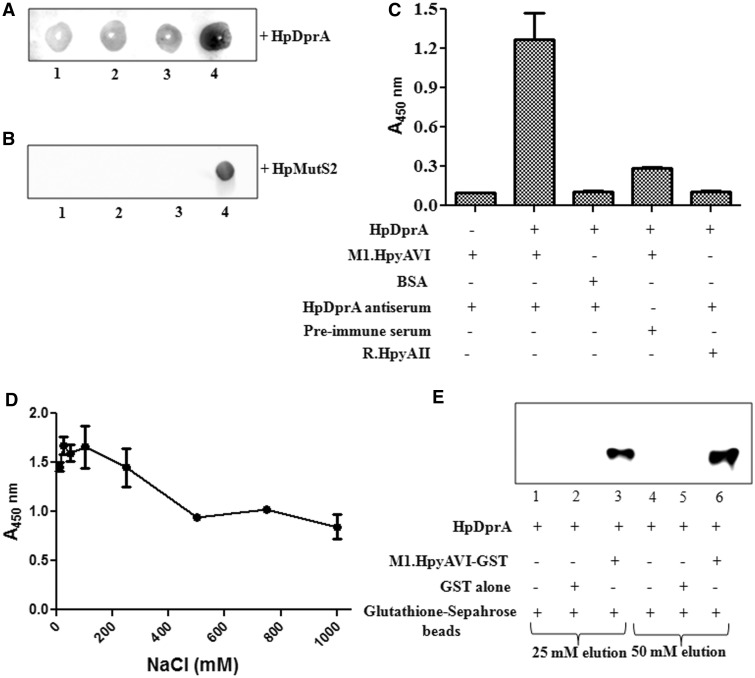
Figure 7.
Analysis of in vitro interaction of M.HpyAVIA with HpDprA. (A) Far western analysis of interaction between HpDprA and M.HpyAVIA. Increasing amount of M.HpyAVIA [0.5, 1.0, 2.0 and 4.0 (µg), lanes 1–4] was immobilized on a nitrocellulose membrane. Interaction was studied by incubation of membrane with 1 µM HpDprA and immunoblotting with HpDprA antiserum to detect any bound HpDprA on the membrane. (B) Far western analysis of interaction between HpMutS2 and M.HpyAVIA. Increasing amount of M.HpyAVIA [0.5, 1.0, 2.0 (µg), lanes 1–3] was immobilized on a nitrocellulose membrane. Interaction was studied by incubation of membrane with 1 µM HpMutS2 followed by immunoblotting with HpMutS2 antiserum. Lane 4: 2.0 µg of HpMutS2 immobilized on membrane as positive control. (C) ELISA-based protein–protein interaction assay. ELISA plates coated with M.HpyAVIA and blocked with 10% skimmed milk in PBS were incubated with HpDprA. The remaining steps of reaction were performed as described in ‘Materials and Methods’ section. (D) Effect of salt on HpDprA–M.HpyAVIA interaction. M.HpyAVIA (2 µg) was immobilized on ELISA plates and incubated with 1 µM HpDprA in presence of increasing concentrations of NaCl [10, 25, 50, 100, 250, 500, 750 and 1000 (mM)]. (E) Interaction between HpDprA and M.HpyAVIA by co-elution. HpDprA (25 µg) was incubated with GST (25 µg)-bound glutathione beads (lanes 1 and 4), with glutathione beads alone (lanes 2 and 5) or with GST-tagged M.HpyAVIA (25 µg)-bound glutathione beads (lanes 3 and 6). Lanes 1–3 represent elution with 25 mM glutathione. Similarly, lanes 4–6 represent elution with 50 mM glutathione.