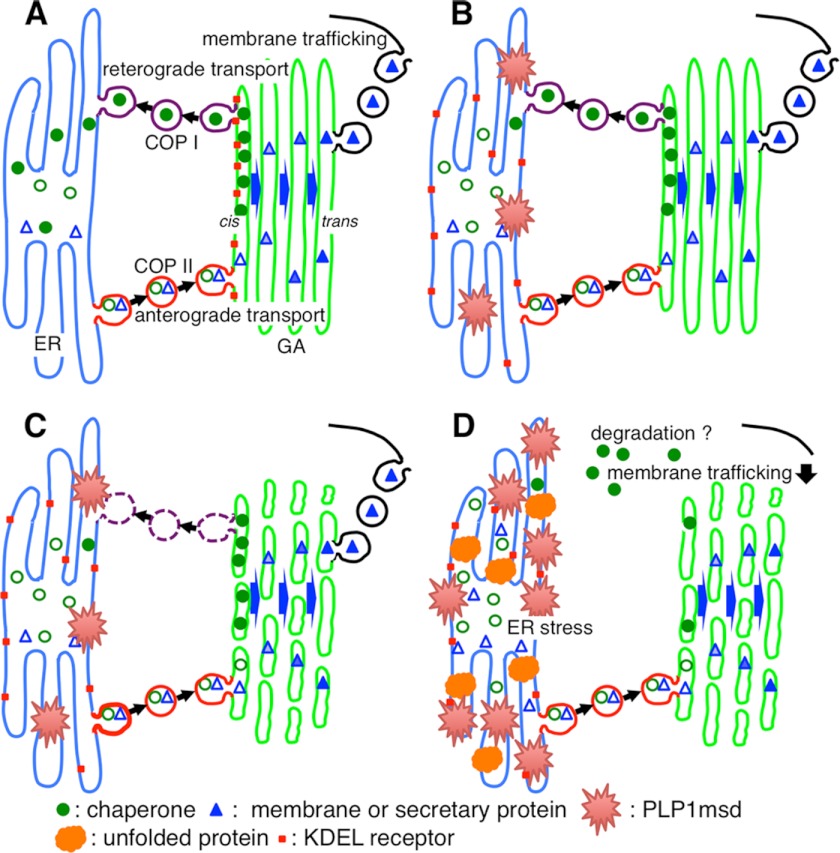
FIGURE 10.
Scheme for the mechanism of ER chaperone depletion and GA fragmentation. A, under physiological conditions, efficient amounts of mature ER chaperones (filled circles) can fold unfolded proteins into their correct conformation. Immature ER chaperones with KDEL motifs (open circles), along with membrane and secretory proteins (triangles), are first transported from ER exit sites to the entry (cis) side of the GA to undergo post-translational modifications (coatomer protein (COPII) II-mediated anterograde transport). The ER chaperones are recycled back to the ER through the interaction with the KDEL receptor (red square) in the GA (coatomer protein I-mediated retrograde transport). In contrast, mature membrane and secretory proteins that reach to the trans-Golgi are further transported to various cellular destinations. B, ER stress-related mutant protein (red spines) induces dysfunction of retrograde transport from the GA to the ER by KDEL receptor mis-localized in the ER, resulting in a reduced supply of ER chaperones. C, this inhibition of retrograde transport, and probably the ER stress itself, may lead to the fragmentation of the GA. D, dysfunction of the ER to GA transport may jam membrane and secretory protein trafficking, leading to the further accumulation of misfolded proteins (orange). These changes in cellular homeostasis triggered by misfolded mutant proteins may further accelerate ER stress.