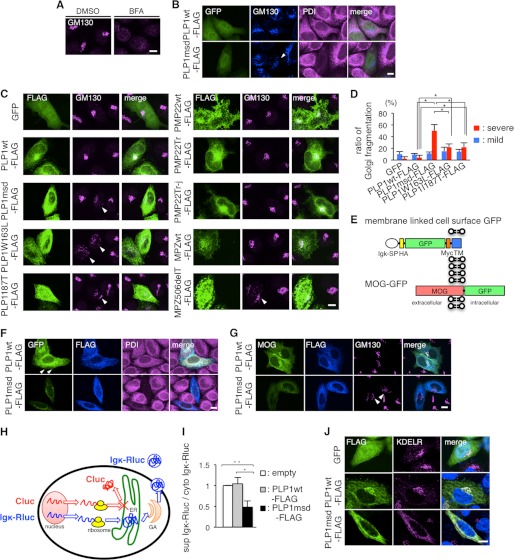
FIGURE 9.
PLP1msd overexpression induces GA fragmentation, retention of MOG in the ER, and reduction of protein secretion. A and B, immunocytochemistry of GM130 in HeLa cells treated with BFA (A) and HeLa cells expressing PLP1wt or PLP1msd (B). HeLa cells were treated with DMSO (as a control) or BFA as described in the legend to Fig. 7A. HeLa cells co-transfected with GFP (to visualize transfected cells) along with PLP1wt-FLAG or PLP1msd-FLAG were immunostained using anti-GM130 (blue) and anti-PDI (magenta) antibodies and were observed with a confocal fluorescence microscope. Cells expressing PLP1msd showed the fragmentation of the GA (arrowhead). Scale bar, 10 μm. C, immunocytochemistry of GM130 in HeLa cells expressing PLP1, PMP22, and MPZ mutants. HeLa cells transfected with the indicated vectors were immunostained with the anti-FLAG (green) and anti-GM130 (magenta) antibodies. Note that cells expressing PLP1 mutants and MPZ506delT showed fragmentation of GA detected by GM130 staining (arrowheads). Scale bar, 10 μm. D, the proportion of cells showing GA fragmentation in HeLa cells transfected with PLP1wt or mutant PLP1 genes, as shown in B and C. FLAG-positive cells were classified into 3 categories based on GA morphology, as normal, mild fragmentation, and severe fragmentation and the number of cells in each class was counted. Data for normal are not shown. The results are represented as the mean ± S.E. from three independent experiments with >100 cells counted in each experiment (*, p ≤ 0.05; **, p ≤ 0.005). E, scheme for the construction of membrane-linked cell surface GFP and MOG-GFP. SP, signal peptide; Igκ, immunoglobulin κ light chain; HA, hemagglutinin; TM, transmembrane of platelet-derived growth factor receptor. F and G, subcellular localization of membrane-linked cell surface GFP (F) or MOG-GFP (G) in HeLa cells expressing PLP1wt-FLAG or PLP1msd-FLAG. HeLa cells co-transfected with membrane-linked cell surface GFP or MOG-GFP along with PLP1wt-FLAG or PLP1msd-FLAG were immunostained using anti-FLAG (blue) and anti-PDI (magenta) antibodies. Cells expressing PLP1wt showed the cell surface GFP expression (arrowhead). Scale bar, 10 μm. H, scheme for the luciferase reporter assay. Secretary Renilla luciferase (Igκ-Rluc, blue), which is fused with Igκ signal peptide, penetrates into the ER and is secreted to the extracellular space through the GA. Firefly luciferase (Cluc, red), which is a cytosolic protein, served as an internal control. This system enables the measurement of secretion of the secretory reporter protein in live cells by comparing the total and supernatant activities of Igκ-Rluc. Co-transfection with the Cluc gene not only detects leakage of these reporter proteins to the supernatant from dead cells, but also compares translation efficacies between cytoplasmic and secretary proteins. I, luciferase reporter assay to evaluate the effect of PLP1msd on the secretory protein transport. HeLa cells were co-transfected with the firefly luciferase (Cluc) and Igκ-Rluc genes along with an empty vector, PLP1wt-FLAG, or PLP1msd-FLAG. Each of Cluc and Igκ-Rluc in the cell lysate and supernatant was simultaneously measured by a luminometer. Efficient of secretion of Igκ-Rluc was calculated as follows: Renilla luciferase activity of supernatant/total (supernatant plus cytosol) Renilla luciferase activity. Results are represented as fold-induction compared with empty vector control experiment. Values are represented as the mean ± S.E. from three independent experiments (*, p ≤ 0.05; **, p ≤ 0.005). J, immunocytochemistry of KDEL receptor in HeLa cells expressing PLP1wt or PLP1msd. HeLa cells transfected with the indicated vectors were immunostained with the anti-FLAG (green) and anti-KDEL receptor (magenta) antibodies and observed with a confocal fluorescence microscope. Scale bar, 5 μm.