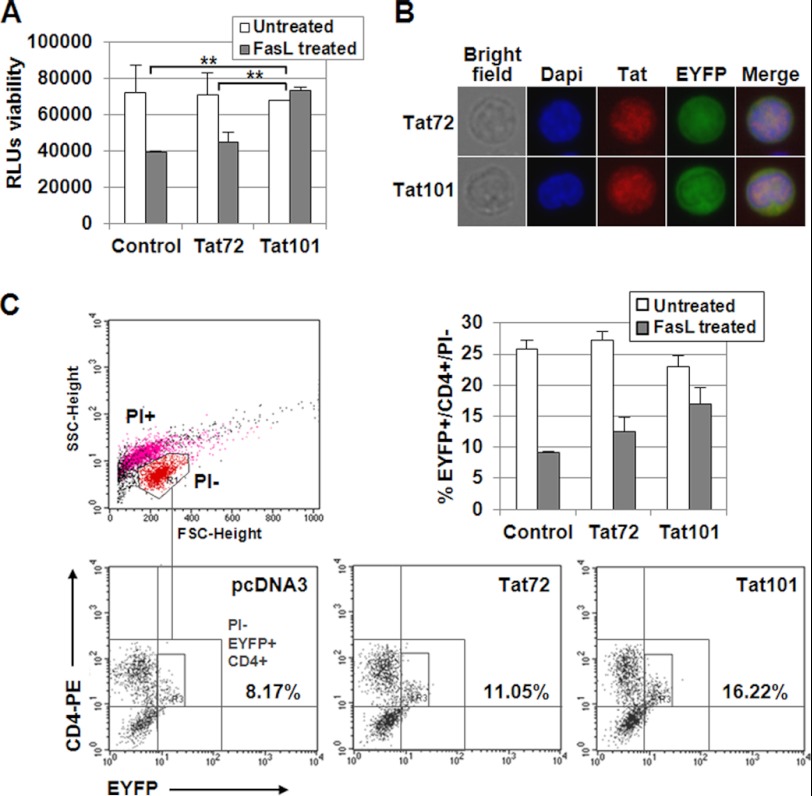
FIGURE 1.
FasL-mediated apoptosis was reduced in PBLs expressing intracellular Tat101. A, resting PBLs were transiently transfected with pCMV-Tat72 or pCMV-Tat101 expression vectors or with pcDNA3 as negative control. pEYFP-C1 was used as control of transfection efficiency. PBLs were maintained for 3 days without stimulus, and then were treated with FasL for 18 h. Viability was measured by chemiluminescence. The bar diagram represents the media of three independent experiments, and lines on top of the bars correspond to the mean ± S.E. Two-way ANOVA with Bonferroni pos-test analysis was performed for statistical analysis; ** indicates p < 0.01. B, Tat expression and nuclear localization were confirmed by immunofluorescence. DAPI was used for nuclear staining. C, resting PBLs were transiently transfected with pCMV-Tat72 or pCMV-Tat101 expression vectors or with pcDNA3 as negative control. pEYFP-C1 was used as control of transfection efficiency. PBLs were maintained for 3 days without stimulus and then were treated with FasL for 18 h. Cells were stained with a monoclonal antibody against CD4 conjugated with PE and then with PI. Signals corresponding to EYFP, PE, and PI were analyzed by flow cytometry in FL1, FL2, and FL3 channels, respectively. SSC/FSC dot plot was used to select living (PI−) cells (region R1) as follows: red events correspond to PI−, living cells; magenta events correspond to PI+, dead cells; black events correspond to cellular debris. The numbers in the PI−/EYFP+/CD4+ dot plots show the percentage of CD4+/EYFP+ cells within the PI− region (region R3). The bar diagram represents the media of three independent experiments, and the lines on top of the bars correspond to the mean ± S.D.