FIGURE 3.
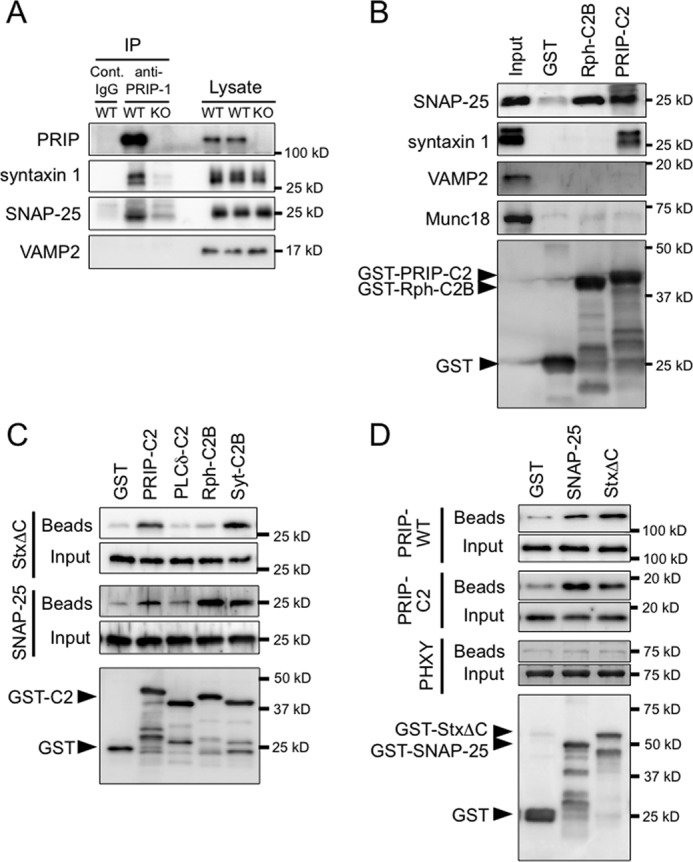
Interaction of PRIP-C2 with SNARE proteins. A, PRIP-1 was immunoprecipitated (IP) using mouse anti-PRIP-1 monoclonal antibody or normal IgG (Cont. IgG) from brain lysates of PRIP-WT and PRIP-KO mice. The lysates and immunoprecipitates were subjected to Western blotting with antibodies against the proteins indicated on the left. 2 or 20% of the total amount of brain lysates or immunoprecipitates, respectively, was applied to SDS-PAGE. Syntaxin 1 was seen in double bands; the lower band would be a degraded product. B, GST or GST-fused C2 domains (PRIP-C2 and Rph-C2B) immobilized on glutathione beads were mixed with PC12 cell lysate, followed by extensive washing, and the bound proteins were analyzed by Western blotting using the antibodies indicated on the left. The bottom panel indicates the blot of the beads, probed with anti-GST antibody. GST-C2 proteins (PRIP-C2 and Rph-C2B) appear as the top band in each lane based on the expected molecular size as indicated by an arrowhead with some degraded proteins below the band. C, the mixture of 100 pmol each of GST or GST-fused C2 domains (PRIP-C2, PLCδ-C2, Rph-C2B, and Syt-C2B) and SNAP-25 or syntaxin 1ΔC (StxΔC) were incubated at 4 °C for 3 h and then applied to glutathione beads. After another hour incubation, the beads were washed extensively followed by Western blotting. SNAP-25 and StxΔC bound to the beads were probed with anti-SNAP-25 and anti-syntaxin 1 antibodies, respectively. The bottom panel indicates the blot of the beads mixed with SNAP-25, which was probed with anti-GST antibody. GST-C2 protein appears as the top band in each lane with some degraded proteins below the band. The identical result was obtained for the amount of immobilized GST proteins from the beads mixed with StxΔC. A band in the lane of PLCδ-C2 detected by anti-SNAP-25 antibody was also detected in the control beads at a similar density, indicating that nonspecific, background interaction. D, GST pulldown assay was performed in the reverse direction shown in C. GST, GST-SNAP-25, or GST-StxΔC was mixed with purified PRIP-WT, PRIP-C2, or PHXY (PRIP-1 lacking the C2 domain; see Fig. 1), and then bound proteins on the beads were analyzed as in B using anti-PRIP-1 polyclonal antibody, which recognizes all PRIP-1 constructs. Three independent experiments were performed with similar results.
