FIGURE 8.
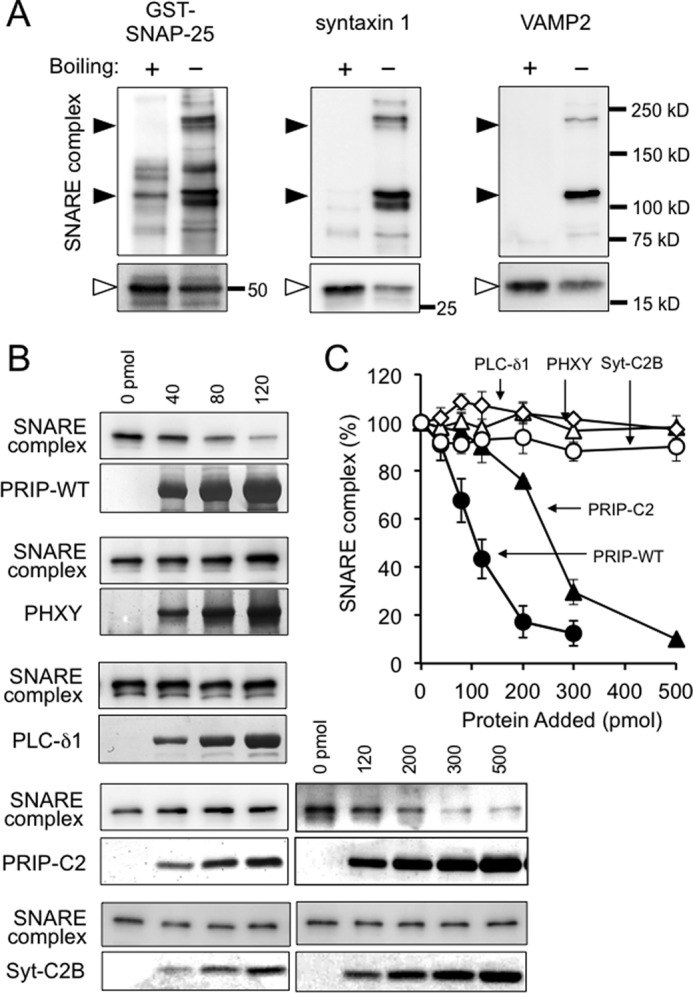
Effect of PRIP on SDS-resistant SNARE complex formation. A, mixture of the purified recombinant proteins of His-VAMP2ΔC, syntaxin 1ΔC, and GST-SNAP-25 was incubated overnight at 4 °C and then subjected to SDS-PAGE with or without boiling after the addition of sample buffer, followed by Western blotting using the indicated antibodies. The black arrowheads indicate high molecular mass bands observed only in the unboiled sample, whereas open arrowheads indicate the bands with the expected molecular masses of monomeric proteins. B, mixture of the three SNARE proteins were incubated for 3 h at 4 °C in the presence or absence of the indicated concentrations of purified wild type PRIP-1 (PRIP-WT), deleted PRIP-1 mutants (PHXY and PRIP-C2), PLC-δ1, or deleted synaptotagmin I mutant (Syt-C2B), followed by Western blotting without boiling. Only the bands of 220 kDa detected with anti-VAMP2 antibody are shown in the figure, but the bands of 110 kDa behaved similarly with 220-kDa bands detected with either antibody. C, the amount of SNARE complex formed shown in B was expressed as relative to that in the absence of PRIP, PLC-δ1, or Syt-C2B. All of the data are the means ± S.E. of four experiments. Closed circle, PRIP-WT; closed triangle, PRIP-C2; open triangle, PHXY; open diamond, PLC-δ1; open circle, Syt-C2B.
