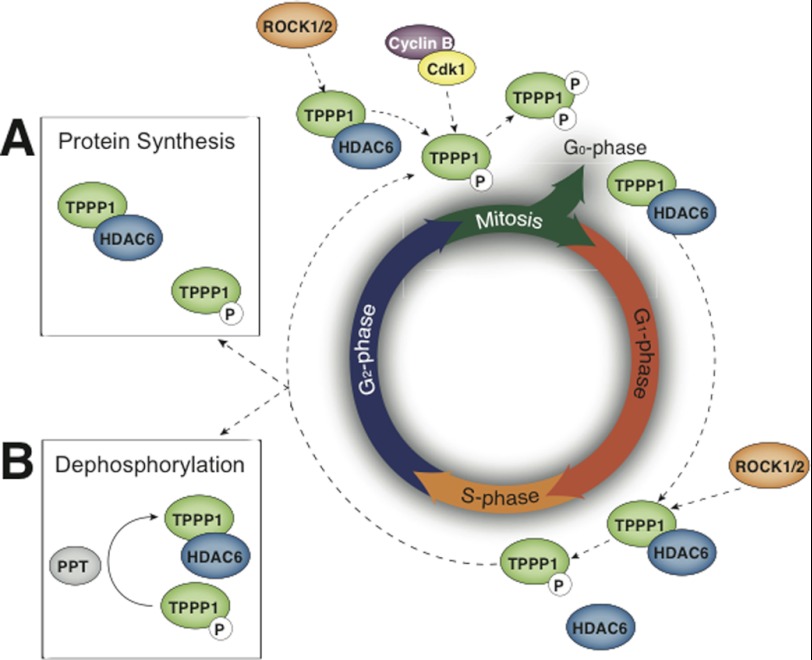
FIGURE 8.
A proposed model of the dynamic phosphorylation of TPPP1 and its activation/deactivation during the cell cycle. TPPP1 phosphorylation by ROCK during late G1-phase inhibits its HDAC6 regulatory activity to decrease MT acetylation and enable cells to enter and/or progress through S-phase. TPPP1 phosphorylation by ROCK and Cdk, which inhibits its HDAC6 regulatory and MT polymerizing activity, respectively, is necessary for cells to exit mitosis and re-enter the G1-phase. It was previously suggested that an increase in MT acetylation is required for mitosis. Therefore, we propose that TPPP1-mediated inhibition of HDAC6 activity, resulting in increased MT acetylation, occurs during the G2-phase. We suggest two alternative models whereby the TPPP1 pool is phosphorylated or partially phosphorylated at late G1-phase by ROCK to permit cells transition into S-phase. This is followed by the synthesis of new TPPP1 protein (A) or its dephosphorylation by a phosphatase (PPT) (B) during the G2-phase to re-establish its inhibition of HDAC6 activity and increase MT acetylation to form a stable mitotic apparatus.