FIGURE 9.
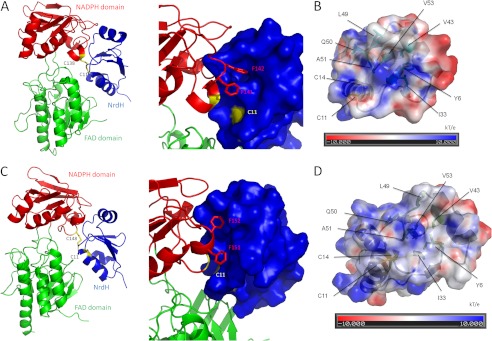
Cg (A and B) and Mt (C and D) NrdH-redoxin have a TrxR binding site near their active site CXXC motif. A ribbon model of the complex between Cg_NrdH-redoxin and Ec_TrxR (A) and of the complex between Mt_NrdH-redoxin and Mt_TrxR (C) are shown. NrdH-redoxin is in blue, and the NADPH domain and FAD domain of TrxR are in red and green, respectively. The interface between both proteins has been enlarged, and the surface representation of NrdH-redoxin is shown. The two Phe residues of TrxR (red sticks) fit in the hydrophobic pocket of NrdH-redoxin. The active site cysteines of TrxR and NrdH-redoxin are in yellow. The residues forming the hydrophobic pocket in Cg (B) and Mt (D) NrdH-redoxin are conserved in both proteins. The electrostatic surface potential of the NrdH-redoxins is shown. The electrostatic potential is given from −10 (red) to 10 (blue) kT/e with k the Boltzmann constant, T the temperature, and e the elementary charge (charge of a proton). Areas with a negative electrostatic potential are colored in red, whereas areas with a positive electrostatic potential are shown in blue. The residues forming the hydrophobic pocket and the active site cysteines are annotated.
