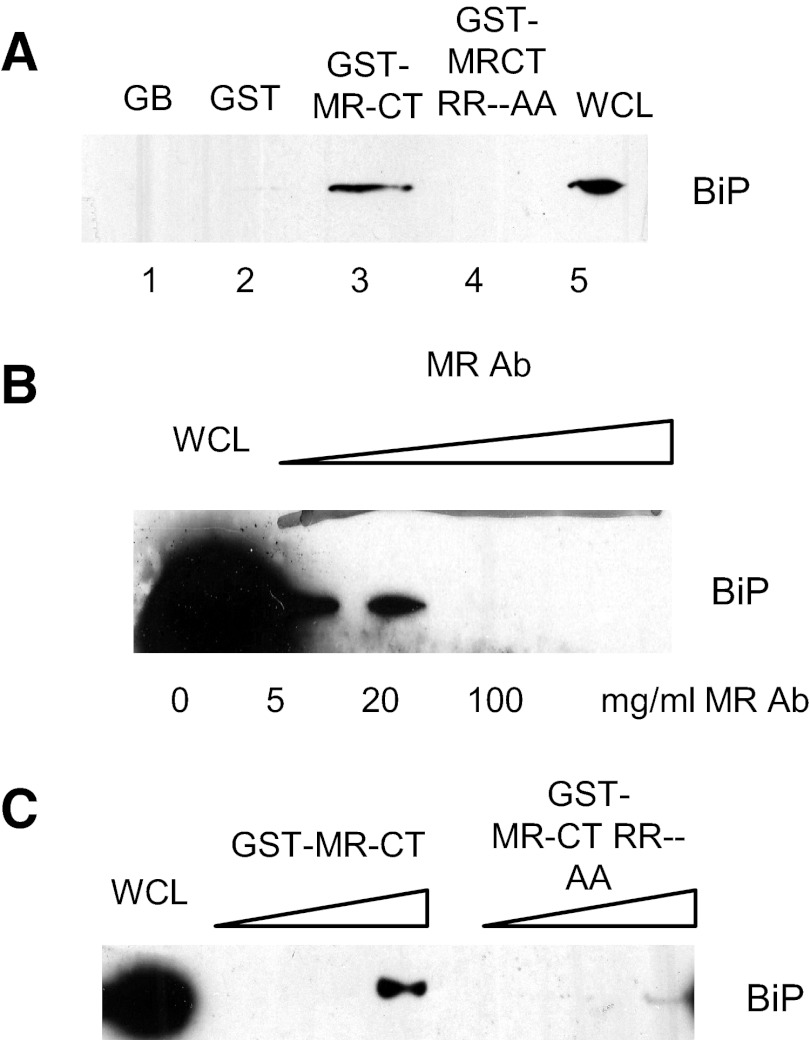
Figure 6. Binding of BiP to the cytoplasmic tail region of the MR.
The coding region of the MRCT from the opti-MR cDNA was subcloned into the pGEX-5X-1 plasmid to generate GST-fused MRCT. Bacterial-expressed GST-MRCT or GST-alone proteins were purified by incubation with GBs. GB-bound GST-MRCT or GST alone was incubated with HeLa cell lysates overnight at 4°C and proteins separated by SDS-PAGE. Western blot analysis showed that BiP bound to GST-MRCT constructs (A, lane 3) but not to GST alone (A, lane 2). To determine specificity of BiP binding to the MRCT, HeLa cell extracts were incubated with GST-MRCT in the presence of increasing concentrations of anti-MR antibody (0–100 μg/ml). Proteins were separated by SDS-PAGE and bound BiP analyzed by Western blot (B). To determine the potential motifs in the MRCTs that bind BiP, the RR amino acids in the KKRR sequence were mutated to AA, and the resulting, mutated MRCT was subcloned into pGEX-5X-1 to generate the GST-MRCT RRAA construct. GST-MRCT-RRAA or GST-MRCT bound to glutathione beads was incubated with HEK293 cell lysates, and BiP binding was examined by Western blot analysis. As shown in A, lane 4, mutation of the RR residues eliminated BiP binding MR tail. To further examine the role of the RR sequence in BiP binding, HeLa lysates were incubated with increasing concentrations of GST-MRCT or GST-MRCT RRAA, and proteins bound to glutathione beads were analyzed by SDS-PAGE and Western blot. As shown in C, BiP binding to the mutated MRCT was reduced significantly. WCL, Whole cell lysates.