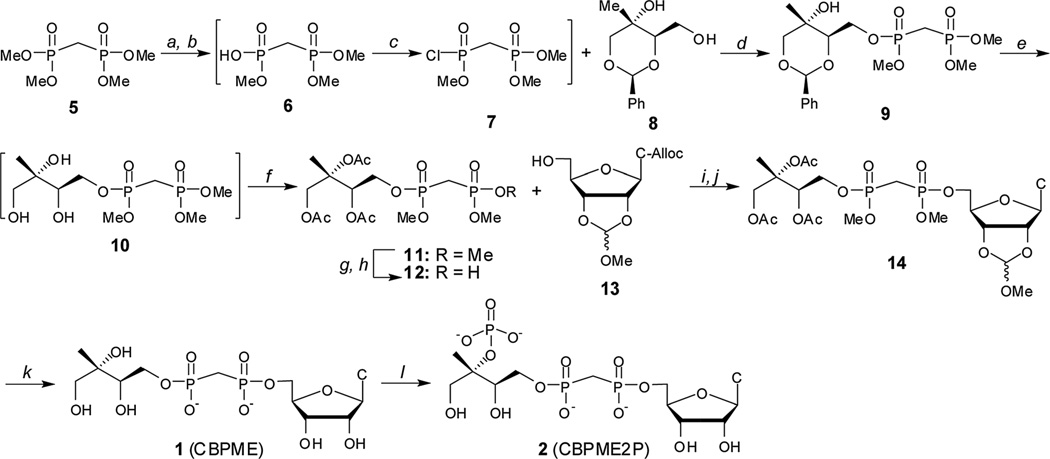
Scheme 1.
Synthesis of stable substrate analogs 1 and 2. Conditions: (a) PhSH, iPr2Net, 12–14 h; (b) DOWEX 50WX8 H+ form; (c) (CH3)2C=C(Cl)N(CH3)2, CH2Cl2, 40 °C; (d) iPr2Net, DMAP, 51–65% over 3 steps; (e) Pd–C, H2 (g), MeOH: (f) Ac2O, iPr2NEt, DMAP, 72–82% over 2 steps; (g) PhSH, Et3N; (h) DOWEX 50WX8 H+ form, 76%; (i) Ph3P, DIAD, THF, 62–78%; (j) PD(Ph3P)4, pTSO2Na, THF/ddH2O, 88–94%; (k) (i) PhSH, Et3N, DMF, 70 °C, 9d, (ii) MeOH/H2O, pH 2.0, 24 h, (iii) 2 : 1 : 0.5 MeOH : H2O : Et3N, 18 h, 39%; (l) IspE, ATP, PEP, PK, 1 h, 43%.