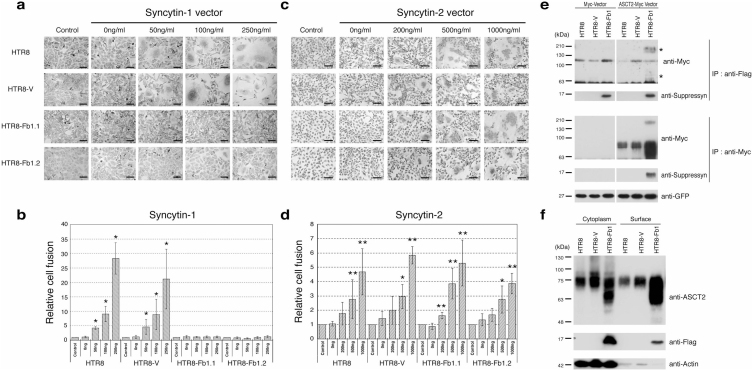
Figure 3. Suppressyn inhibits syn1- but not syn2-induced trophoblast cell fusion and suppressyn binds to the syn1 receptor ASCT2.
HTR8 trophoblast cells were stably transfected with vectors driving the expression of Fb1 (HTR8-Fb1.1 and 1.2) or vector alone (HTR-V). Cells were then transiently transfected with increasing amounts of a vector driving expression of syn1 (a) or syn2 (c), counterstained with hematoxylin and analyzed using phase contrast microscopy. To calculate fusion indices, cells were resuspended and analyzed by flow cytometry (b and d). Relative cell fusion was defined as the number of fused syn1- or syn2-transfected cells (gated by flow cytometry) divided by the number of fused cells in parent (HTR8) and in stable vector- or Fb1-transfected (HTR8-V, HTR8-Fb1.1, HTR8-Fb1.2), but syncytin non-transfected, cells cultured under identical conditions (indicated as controls for each cell line, *p < 0.05 **p < 0.01 when compared to non-transfected controls). Statistical comparisons were made using Kruskal-Wallis and Mann-Whitney U-testing with Bonferroni corrections. Data in (b) and (d) are representative of three independent experiments performed in duplicate. (e) HTR8 parental cells were stably transfected with vectors driving expression of flag-tagged Fb1 and then transiently transfected with a myc-vector or with a vector driving the expression of myc-tagged ASCT2. For the transfection internal control, the pAcGFP1-C1 vector was co-transfected with the myc tag vector and GFP protein expression quantitated using a monoclonal anti-GFP antibody. Lysates were immunoprecipitated with an anti-myc tag antibody and protein-G agarose or anti-flag-M2 agarose. Immunoprecipitants were separated by PAGE and immunoblotted with a myc-specific antibody. *Co-precipitated high and low molecular weight ASCT2. (f) HTR8, HTR8-V and HTR8-Fb1 cells were surface biotinylated and proteins purified using the Pierce cell surface protein isolation kit. Equal amounts of cytoplasmic and biotinylated surface protein were subsequently analyzed with standard SDS-PAGE and immune detection. Data in (e) and (f) are representative of three independent experiments.