Abstract
OBJECTIVES
Implantation of an annuloplasty ring is an essential component of a durable mitral valve repair. Currently available off-the-shelf rings still do not cover all the variations in mitral annulus anatomy and pathology from subject to subject. Computed tomography (CT) and echo imaging allow for 3-D segmentation of the mitral valve and mitral annulus. The concept of tailored annuloplasty rings has been proposed although, to date, no surgically applicable implementation of patient-specific annuloplasty rings has been seen. The objective of this trial was to prove the concept of surgical implantation of a model-guided, personalized mitral annuloplasty ring, manufactured based on individual CT-scan models.
METHODS
ECG-gated CT angiography was performed in six healthy pigs under general anaesthesia. Based on the individual shape of the mitral annulus in systole, a customized solid ring with integrated suturing holes was designed and manufactured from a biocompatible titanium alloy by a rapid process using laser melting. The ring was implanted three days later and valve function was assessed by intraoperative echocardiography. The macroscopic annulus–annuloplasty match was assessed after heart explantation.
RESULTS
CT angiography provided good enough image quality in all animals to allow for segmentation of the mitral annulus. The individually tailored mitral rings were manufactured and successfully implanted in all pigs. In 50%, a perfect matching of the implanted ring and the mitral annulus was achieved. In one animal, a slight deviation of the ring shape from the circumference was seen postoperatively. The rings implanted in the first two animals were significantly oversized but the deviation did not affect valve competence.
CONCLUSIONS
CT image quality and accuracy of the dimensions of the mitral annulus were sufficient for digital modelling and rapid manufacturing of mitral rings. Implantation of individually tailored annuloplasty rings is feasible.
Keywords: Cardiac surgery, Animal feasibility study, Mitral valve repair, Personalized annuloplasty
INTRODUCTION
Mitral valve repair is the gold standard treatment for mitral valve regurgitation [1, 2]. Various repair techniques, including leaflet resection and chord replacement, are used in combination with the implantation of an annuloplasty ring. Mitral annuloplasty is an essential component of a durable mitral valve repair and provides very good mid- and long-term results [3–5]. A number of off-the-shelf rings are available for different underlying pathologies. Saddle-shaped rigid or semi-rigid rings are most commonly used for the repair of primary (degenerative) mitral valve insufficiency. The GeoForm ring or the ETlogix rings (both by Edwards Lifesciences Corp., Irvine, CA, USA) are intended to treat patients with secondary (functional) mitral valve regurgitation. All rings partly influence and remodel the three-dimensional shape of the mitral valve annulus in different ways, but do not take account of anatomical variations between individuals.
Based on the availability of 3-D imaging modalities with sufficiently high resolution and accuracy for modelling the mitral annulus, the concept of patient-specific annuloplasty rings was proposed by Lantada and colleagues [6]. This group designed a ring based on dimensional data obtained from computed tomography (CT) images. The ring was manufactured out of epoxy resin using a rapid-prototyping technique. The rings were not intended for implantation, since the material was not biocompatible and no sutures could be placed through the ring because of its solid composition. In view of these limitations, we sought to develop a personalized, implantable ring using a biocompatible material and including features that facilitate implantation. Here we present a feasibility study where custom-made annuloplasty rings, individually designed, based on CT segmentations of the mitral annulus, were manufactured using a biocompatible titanium alloy and implanted in pigs.
METHODS
Study subjects
Six healthy pigs with a mean weight of 59.5 ± 2.7 kg were included. All pigs underwent a cardiac CT-scan under general anaesthesia. After tailoring and manufacturing of the individualized annuloplasty rings (see below), all animals were re-anaesthetized and operated 3 days later. Immediately after successful ring implantation and assessment of the valve function, they were euthanized and the explanted hearts studied. The protocol was the same for all six animals and the experiment was intended as a proof of concept. All animals received humane care in compliance with the Swiss Animal Protection Law (TSchG) and the Swiss Animal Protection Act (TSchV). The study protocol was approved by the local Committee for Experimental Animal Research (Kantonales Veterinäramt des Kantons Zürich, permission number 008/2011).
Imaging and modelling
The pigs were anaesthetized and ECG-gated CT angiography (Siemens Somatom Definition Flash, Siemens Healthcare, Forchheim, Germany) was performed with 400–500 slices of 0.3 × 0.3 × 0.3 mm3 resolution and the use of 80 ml of Ultravist 300 contrast agent. Beta-blockers and Lidocaine were administered as needed to stabilize and decrease the heart rates of the pigs to 60–70 bpm.
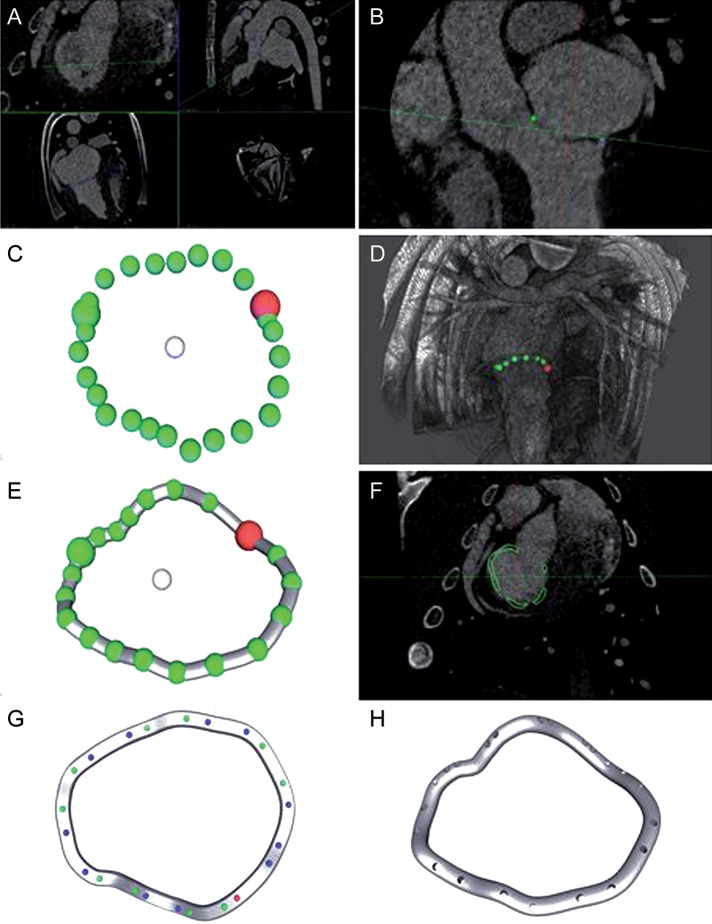
Using these CT images, specially developed software created a digital model of the individual mitral annuli. The principal steps of the modelling process are depicted in Fig. 1. In order to segment the mitral annulus, the software was used to localize the mitral commissures and 22 additional points on the mitral annulus. A three-dimensional model was computed as a cyclic third-order interpolant through all 24 points. This curve served as an initial centreline for the annuloplasty ring geometry. This geometry was adjusted manually before defining the positions of the suturing holes. The software ensured that the distance between holes was between 7 mm and 12 mm; indentations marking the commissures—and thus facilitating orientation during implantation—were automatically added on the atrial surface of the 3-D models (Fig. 2).
Figure 1:
Process of ring manufacturing. (A) Cardiac CT images (0.3 × 0.3 × 0.3 mm3 resolution, end systolic); (B) manual placement of 24 landmarks on the mitral annulus; (C) 3-D shape of the mitral annulus before and (D) after smoothing; (E) computed model of annuloplasty ring following the modelled annulus; (F) ring model shown in CT images; (G) assignment of suturing holes; (H) final ring model.
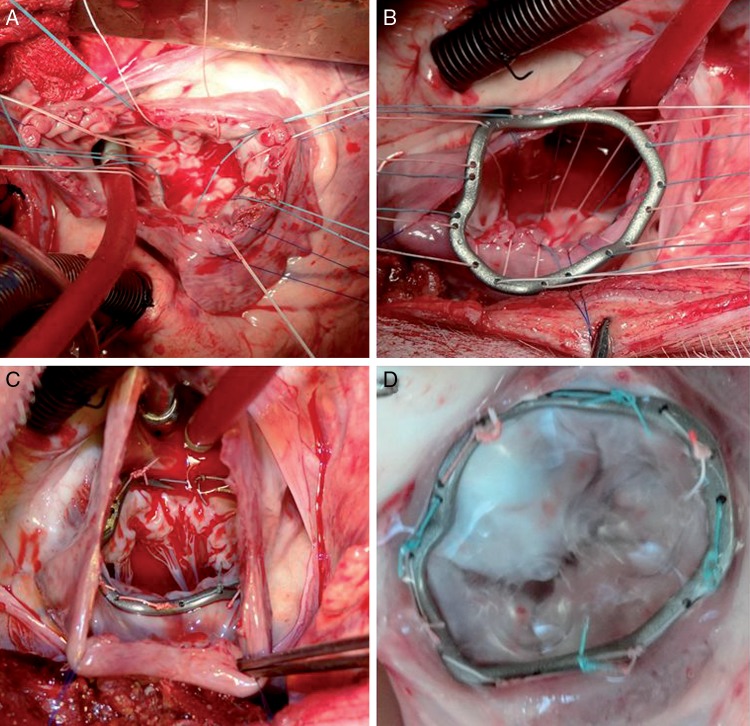
Figure 2:
Ring implantation. (A) Atrial view on mitral valve; (B) preparation of sutures; (C) implanted ring; (D) explanted annulus with ring.
Ring production
Based on these 3-D models, tailored annuloplasty rings (Fig. 3) were produced from a titanium alloy for each pig, using the Selective Laser Melting (SLM) method [7]. SLM is an additive manufacturing process that uses a high-powered laser beam, guided by a digital 3-D model, to create three-dimensional metal parts by locally heating a metal powder above its melting temperature and fusing it. The Ti6Al4V titanium alloy that was used is known to be biocompatible [8]. The process parameters (laser energy, melting time, curing time and afterglow) were optimized to reduce air pockets and maximize the homogeneity and mechanical durability of the material after melting. Homogeneity and air inclusion were assessed visually using microsections of material samples. Durability was tested using repetitive tensile tests.
Figure 3:
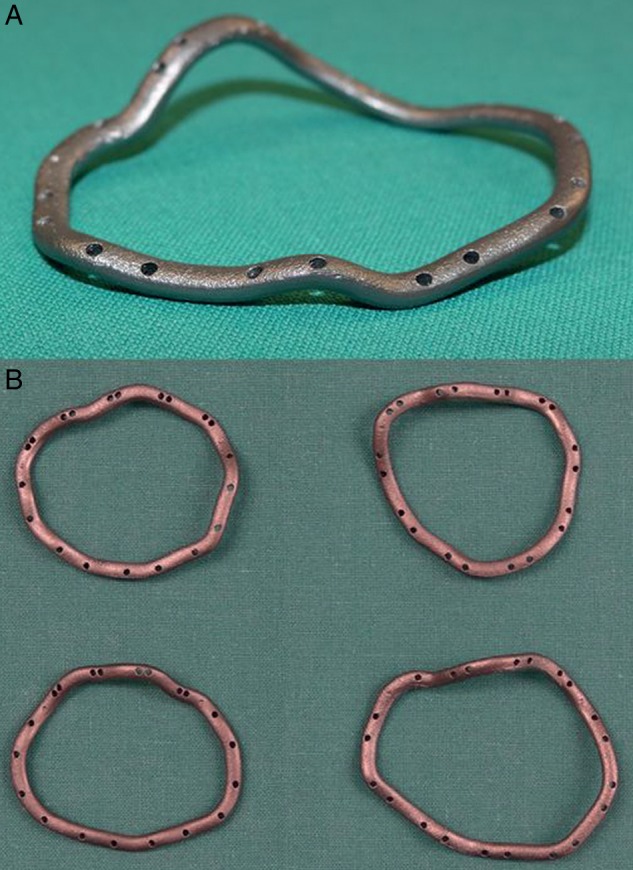
(A) Custom-made ring for Pig 5; (B) comparison between four rings manufactured for Pigs 3–6.
Implantation
The rings were implanted under general anaesthesia using standard haemodynamic monitoring. Access was gained through a left-sided thoracotomy. Cardiopulmonary bypass (CPB) was installed with an arterial cannula in the descending aorta and a venous cannula in the pulmonary artery. The procedure was performed on the beating heart. The left atrium was opened and the mitral valve exposed in a standard way. The ring was implanted using single-knot sutures through the preformed holes (Fig. 2B). A sealing test indicated valve sufficiency. After closure of the left atrium, the animals were weaned off bypass and valve function was assessed by epicardial B-mode and Doppler echocardiography (Philips iE33 platform and X5-S real-time probe, Philips Medical Systems, Andover, MA, USA). Afterwards the animals were euthanized, the hearts explanted and the mitral annuli with the implanted rings dissected and examined for prosthesis-annulus alignment (Fig. 2C).
RESULTS
Imaging, modelling and production
Identification of the mitral annulus in the CT angiography images and ring modelling was possible in all cases. Manufacturing of the rings took three days. The precision of the melting process preserved all relevant details of the ring models, including the preformed suturing holes and commissural indentations. The mean weight of the rings was 2.2 ± 0.2 g. The mean perimeter of the rings was 109.7 ± 11.2 mm. The mean diameter from commissure to commissure was 32.4 ± 5.2 mm. Eight to twelve suturing hole pairs were preformed and two indentations were created, corresponding to the commissures (Table 1). The computed annular geometry and the rings created from these data differed considerably in shape from commercially available mitral annuloplasty rings (Fig. 3).
Table 1:
Odds ratio (OR) and ring parameters
| Pig | A-Weight | OR-T | CPB-T | Weight | Diameter | Post. P | Ant.P | Peri. | Stitches | Distance |
|---|---|---|---|---|---|---|---|---|---|---|
| P1 | 59.5 | – | – | 2.3 | 36.9 | 68.2 | 49 | 117.2 | 11 | 8 |
| P2 | 60 | 100 | 70 | 2.43 | 39.2 | 66 | 57.6 | 123.6 | 12 | 8 |
| P3 | 60.5 | 60 | 50 | 2.04 | 32.1 | 59.7 | 40 | 99.7 | 9 | 8 |
| P4 | 54.5 | 65 | 40 | 2.04 | 24.7 | 70.6 | 30.4 | 101 | 9 | 6.1 |
| P5 | 62.5 | 120 | 50 | 2.39 | 31.7 | 78.3 | 39.9 | 118.2 | 10 | 8 |
| P6 | 60 | 70 | 40 | 2 | 29.6 | 62.1 | 36.4 | 98.5 | 8 | 7.6 |
P1–P6: labelling of animals; A-Weight: weight of animals in kg; OR-T: OR time in min; CPB-T: cardiopulmonary bypass time in min; Weight: weight of rings in g; Diameter: overall diameter of the ring in mm; Ant. P: perimeter from anterior to posterior commissure around the anterior annulus in mm; Post. P: perimeter from anterior to posterior commissure around the posterior commissure in mm; Peri.: total perimeter of the ring in mm; Stitches: number of stitches; Distance: distance between the suturing holes in mm.
Implantation
Operation time was 83 ± 25.3 min and CPB time was 50 ± 12.2 min. Ring suturing time was comparable to that for conventional annuloplasty rings but no time was required for sizing. Implantation of the individual rings was successful in all six cases. Eight to twelve sutures were necessary for attachment. In the first two animals the rings were significantly oversized; their diameters differed from the annulus around 10 mm in all directions. A system error in the modelling process was identified as a cause for this mismatch and was corrected for the subsequent implantations: the modelling process and software were adjusted accordingly before continuation of the experiment with the remaining four animals. All rings chosen in the second batch (Pigs 3–6) matched the annular circumference almost perfectly (Fig. 2C and D). Epicardial echocardiography after implantation showed no new MR and normal movement of both leaflets.
DISCUSSION
Mitral valve repair is considered the gold standard for the treatment of mitral valve regurgitation [1, 2]. Annuloplasty rings were introduced in 1971 to achieve stabilization and reduction of the mitral annulus [9]. Ring shapes underwent continuous development, from planar oval rings over planar D-shaped and later saddle-shaped rings. The rationale for this evolution is the attempt to increase the postoperative curvature of the mitral leaflets to reduce peak leaflet stress and to achieve a larger area of coaptation [10, 11]. Additionally, rings have been developed to address the asymmetric geometry of the mitral annulus in special situations such as dilated cardiomyopathy [12]. Compared to conventional (symmetrical) annuloplasty rings, asymmetric designs produce less mechanical stress at the mitral annulus, whilst achieving the same increase of the area of coaptation [13].
Other concepts like flexible or partial rings were introduced with the aim of meeting the requirements of preserving annular expansion throughout the cardiac cycle[14, 15]. Devices that can be adjusted in the beating heart after closure of the atrium are now available, allowing post-procedural fine adjustment (e.g. the Valtech Cardinal adjustable annuloplasty ring: unpublished data).
In medical disciplines like orthopaedics, traumatology and oral and maxillofacial surgery, many research projects have been successfully undertaken, addressing production and implantation of patient-specific implants [16, 17]. In contrast, in cardiac surgery, individual tailoring of devices has not yet been investigated extensively. The difficulties are the soft tissue composition of the heart and the changing dimensions during the cardiac cycle. The mitral valve anatomy is complex and the three-dimensional geometry of the annulus shows high variability between individuals.
The concept of patient-specific annuloplasty rings was first introduced in 2010 [6]. The authors presented an image-based modelling approach that resulted in an annulus model that was printed in epoxy resin, using stereo lithography. The material is not biocompatible and the models were not intended for implantation.
Here we report the first animal experiment where custom-shaped solid annuloplasty rings were successfully implanted in six healthy pigs. The pig as an animal model was chosen because the anatomy of the cardiovascular system, especially the mitral valve, has been shown to be very similar to the human anatomy [18, 19]. The rings were designed according to the individual annular morphology. Unlike in previous approaches, the rings were custom-made from a biocompatible Ti6Al4V titanium alloy in a rapid manufacturing process. The ring design included suturing holes, assuring good fixation at the annulus, and featured commissural markers facilitating the correct ring orientation—an aspect which is of particular importance when dealing with asymmetrical rings.
Image quality and accuracy of CT imaging were sufficient for the digital modelling of the annulus. Nevertheless, the use of 3-D echocardiography may be considered as an alternative approach for further investigations, as this technique allows for studying the mitral annular shape dynamically, compared to the non-dynamic CT assessment. Which moment of the cardiac cycle is best for defining the final shape and size of an individually tailored annuloplasty ring is a subject that remains to be studied.
The chosen design, consisted of a solid, biocompatible titanium alloy ring with inbuilt suturing holes that ensured secure implantation and commissural markers that facilitated positioning of the ring. Suturing times were comparable to suturing times with conventional rings, while no CBP time was wasted on ring sizing.
An important advantage of patient-specific mitral valve annuloplasty might be the possibility of individual treatment optimization, based on quantitative functional models. Modifications of the annuloplasty ring—addressing the individual underlying pathology, including symmetrical or asymmetrical downsizing—might result in a better outcome. However, the proof of such a concept is still lacking.
The present study only investigated the acute feasibility of implantation of custom-made solid metal rings. At this point, no assessment of long-term aspects—such as endothelial healing—has been performed. Also, the mitral valves of the study pigs were competent and hence no statement can be made concerning reduction of mitral regurgitation or valve competence after correctional ring annuloplasty. The next research steps therefore include chronic animal trials, fatigue testing and refinement of the modelling software, as well as studying the efficacy in subjects with mitral regurgitation.
In conclusion, the concept of personalized annuloplasty might offer patient-specific treatment of mitral valve disease according to the individual anatomy and pathology. Planning, production and implantation of individually built, biocompatible annuloplasty rings are feasible in an animal model.
Funding
This work was funded by the Swiss Heart Foundation within its regular program for supporting basic research.
Conflict of interest: none declared.
REFERENCES
- 1.Vahanian A, Alfieri O, Andreotti F, Antunes MJ, Barón-Esquivias G, et al. Authors/Task Force Members. Guidelines on the management of valvular heart disease (version 2012): The Joint Task Force on the Management of Valvular Heart Disease of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS) Eur J Cardiothorac Surg. 2012;42:S1–44. doi: 10.1093/ejcts/ezs455. [DOI] [PubMed] [Google Scholar]
- 2.Markar SR, Sadat U, Edmonds L, Nair SK. Mitral valve repair versus replacement in the elderly population. J Heart Valve Dis. 2011;20:265–71. [PubMed] [Google Scholar]
- 3.Seeburger J, Borger MA, Doll N, Walther T, Passage J, Falk V, et al. Comparison of outcomes of minimally invasive mitral valve surgery for posterior, anterior and bileaflet prolapse. Eur J Cardiothorac Surg. 2009;36:532–38. doi: 10.1016/j.ejcts.2009.03.058. [DOI] [PubMed] [Google Scholar]
- 4.David TE, Ivanov J, Armstrong S, Christie D, Rakowski H. A comparison of outcomes of mitral valve repair for degenerative disease with posterior, anterior, and bileaflet prolapse. J Thorac and Cardiovasc Surg. 2005;130:1242–49. doi: 10.1016/j.jtcvs.2005.06.046. [DOI] [PubMed] [Google Scholar]
- 5.Braunberger E, Deloche A, Berrebi A, Abdallah F, Celestin JA, Meimoun P, et al. Very long-term results (more than 20 years) of valve repair with Carpentier's techniques in nonrheumatic mitral valve insufficiency. Circulation. 2001;104:I8–I11. [PubMed] [Google Scholar]
- 6.Díaz Lantada A, Valle-Fernández RD, Morgado PL, Muñoz-García J, Muñoz Sanz JL, Munoz-Guijosa JM, et al. Development of personalized annuloplasty rings: combination of CT images and CAD-CAM tools. Ann Biomed Eng. 2010;38:280–90. doi: 10.1007/s10439-009-9805-z. [DOI] [PubMed] [Google Scholar]
- 7.Vandenbroucke B, Kruth J. Selective laser melting of biocompatible metals for rapid manufacturing of medical parts. Rapid Prototyping Journal. 2007;13:196–203. [Google Scholar]
- 8.Long M, Rack HJ. Titanium alloys in total joint replacement—a materials science perspective. Biomaterials. 1998;19:1621–39. doi: 10.1016/s0142-9612(97)00146-4. [DOI] [PubMed] [Google Scholar]
- 9.Carpentier A, Deloche A, Dauptain J, Soyer R, Blondeau P, Piwnica A, et al. A new reconstructive operation for correction of mitral and tricuspid insufficiency. J Thorac Cardiovasc Surg. 1971;61:1–13. [PubMed] [Google Scholar]
- 10.Jensen M, Jensen H, Smerup M, Levine R. Saddle-shaped mitral valve annuloplasty rings experience lower forces compared with flat rings. Circulation. 2008;118:250–55. doi: 10.1161/CIRCULATIONAHA.107.746776. [DOI] [PubMed] [Google Scholar]
- 11.Salgo IS, Gorman JH, Gorman RC, Jackson BM, Bowen FW, Plappert T, et al. Effect of annular shape on leaflet curvature in reducing mitral leaflet stress. Circulation. 2002;106:711–17. doi: 10.1161/01.cir.0000025426.39426.83. [DOI] [PubMed] [Google Scholar]
- 12.De Bonis M. The GeoForm annuloplasty ring for the surgical treatment of functional mitral regurgitation in advanced dilated cardiomyopathy. Eur J Cardiothorac Surg. 2011;40:488–95. doi: 10.1016/j.ejcts.2010.11.048. [DOI] [PubMed] [Google Scholar]
- 13.Daimon M, Fukuda S, Adams DH, McCarthy PM, Gillinov AM, Carpentier A, et al. Mitral valve repair with Carpentier-McCarthy-Adams IMR ETlogix annuloplasty ring for ischemic mitral regurgitation: early echocardiographic results from a multi-center study. Circulation. 2006;114:I588–93. doi: 10.1161/CIRCULATIONAHA.105.001347. [DOI] [PubMed] [Google Scholar]
- 14.Durán CMC, Pomar JLJ, Cucchiara GG. A flexible ring for atrioventricular heart valve reconstruction. J Cardiovasc Surg (Torino) 1978;19:417–20. [PubMed] [Google Scholar]
- 15.Odell JA, Schaff HV, Orszulak TA. Early results of a simplified method of mitral valve annuloplasty. Circulation. 1995;92:II150–54. doi: 10.1161/01.cir.92.9.150. [DOI] [PubMed] [Google Scholar]
- 16.Scolozzi PP. Maxillofacial reconstruction using polyetheretherketone patient-specific implants by ‘mirroring’ computational planning. Aesthetic Plast Surg. 2012;36:660–65. doi: 10.1007/s00266-011-9853-2. [DOI] [PubMed] [Google Scholar]
- 17.Shen F, Chen B, Guo Q, Qi Y, Shen Y. Augmented reality patient-specific reconstruction plate design for pelvic and acetabular fracture surgery. Int J Comput Assist Radiol Surg. 2012 doi: 10.1007/s11548-012-0775-5. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 18.Smerup M, Pedersen T, Nyboe C. A long-term porcine model for evaluation of prosthetic heart valves. Heart Surgery Forum. 2004;7:e259–e264. doi: 10.1532/HSF98.20041015. [DOI] [PubMed] [Google Scholar]
- 19.Crick SJS, Sheppard MNM, Ho SYS, Gebstein LL, Anderson RHR. Anatomy of the pig heart: comparisons with normal human cardiac structure. J Anat. 1998;193(Pt 1):105–19. doi: 10.1046/j.1469-7580.1998.19310105.x. [DOI] [PMC free article] [PubMed] [Google Scholar]