Abstract
Aims
We discovered that some adults with coronary heart disease (CHD) have a high density lipoprotein (HDL) subclass which induces human aortic smooth muscle cell (ASMC) apoptosis in vitro. The purpose of this investigation was to determine what properties differentiate apoptotic and non-apoptotic HDL subclasses in adults with and without CHD.
Methods and results
Density gradient ultracentrifugation was used to measure the particle density distribution and to isolate two HDL subclass fractions, HDL2 and HDL3, from 21 individuals, including 12 without CHD. The HDL fractions were incubated with ASMCs for 24 h; apoptosis was quantitated relative to C2-ceramide and tumour necrosis factor-alpha (TNF-α). The observed effect of some HDL subclasses on apoptosis was ∼6-fold greater than TNF-α and ∼16-fold greater than the cell medium. We observed that apoptotic HDL was (i) predominately associated with the HDL2 subclass; (ii) almost exclusively found in individuals with a higher apoC-I serum level and a novel, higher molecular weight isoform of apoC-I; and (iii) more common in adults with CHD, the majority of whom had high (>60 mg/dL) HDL-C levels.
Conclusions
Some HDL subclasses enriched in a novel isoform of apoC-I induce extensive ASMC apoptosis in vitro. Individuals with this apoptotic HDL phenotype generally have higher apoC-I and HDL-C levels consistent with an inhibitory effect of apoC-I on cholesteryl ester transfer protein activity. The association of this phenotype with processes that can promote plaque rupture may explain a source of CHD risk not accounted for by the classical risk factors.
Keywords: High density lipoprotein, Atherosclerosis, Smooth muscle cell, Apoptosis, Apolipoprotein C-I
1. Introduction
A number of large, population-based studies have observed that an elevated high density lipoprotein-cholesterol (HDL-C) level is associated with an attenuated risk of atherosclerosis.1 This antiatherogenic property of HDL is typically ascribed to the ability of HDL to promote reverse cholesterol transport, although this is but one of a number of functions of HDL that reduce coronary heart disease (CHD) risk.2,3 There is a remarkable diversity in the population of HDL particles with respect to the constituent apolipoproteins, proteins, and lipids resulting in multiple subclasses which differ with respect to the particle density, size, and surface charge.4–6 Identification of a specific HDL subclass that is more, or less, cardioprotective than others and thus a more powerful predictor of CHD than total HDL-C has been the subject of numerous investigations, but no consensus has been reached. Although two major subclasses, HDL2 and HDL3, are commonly described in the literature, the number of identifiable subclasses depends on the mode of separation (ultracentrifugation, immunoisolation, gradient gel electrophoresis, nuclear magnetic resonance, etc.) and the inherent resolution of the method. Subclass heterogeneity can be further increased by transient modifications of HDL-associated proteins and lipids as part of the acute phase response in certain disease states (e.g. infection and myocardial infarction) and in more chronic diseases (e.g. diabetes mellitus, atherosclerosis, and rheumatoid arthritis).7,8 These modifications, as well as a myriad of factors which participate in HDL remodelling during the circulating lifetime of the particle population, can result in dysfunctional HDL subclasses that may paradoxically promote rather than protect against CHD. This unparalleled lipoprotein diversity has led to the conclusion that measures of HDL function may better correlate with the CHD risk than the HDL-C level or a singular subclass of HDL.9,10
Evidence supporting the antiatherogenic role of HDL stems, in part, from investigations of how HDL and various HDL subclasses favourably affect many facets of endothelial cell,11–14 vascular smooth muscle cell,15–17 and macrophage foam cell18,19 function notably including anti-apoptotic effects on these cells. In contrast to these antiatherogenic functional properties, Kolmakova et al.20 discovered apoC-I-enriched HDL isolated from pooled cord blood of certain low birth weight (LBW) infants studied by Kwiterovich et al.21 had a remarkable proapototic effect resulting in a 5- to 25-fold enhancement of human aortic smooth muscle cell (ASMC) apoptosis compared with cells incubated with apoC-I-poor HDL. This atherogenic property of apoC-I was substantiated by additional in vivo experiments using a rabbit model to show apoC-I co-localized with caspase-3 and ceramide at the site of atherosclerotic plaque rupture.22 Since ASMCs are essential in maintaining the integrity of the fibrous cap overlaying the atherosclerotic plaque, a protein or complex such as apoC-I-enriched HDL, which induces smooth muscle cell apoptosis, may promote atherosclerotic plaque rupture leading to thrombosis and myocardial infarction.23 This HDL phenotype is distinct from dysfunctional HDLs reported in the literature because the characteristics are not transient and/or mutable as in the case of functional changes in HDL induced by disease processes (i.e. inflammation, infection, etc.). Instead, the apoC-I-enriched HDL appears to be a de novo phenotype with proatherogenic properties.
In normolipemic individuals, apoC-I is primarily carried by HDL in contrast to hypertriglyceridemic subjects where it is primarily carried by very low-density lipoproteins (VLDL).24 Experiments by Gautier et al.25 revealed pure apoC-I (i.e. apoC-I not associated with a lipoprotein particle) are the most potent endogenous inhibitor of cholesteryl ester transfer protein (CETP) in humans. Dumont et al.26 subsequently showed that apoC-I-rich HDL is a stronger inhibitor of CETP than apoC-I-poor HDL. Subjects with apoC-I-enriched HDL will therefore have a higher HDL-C level. In addition, more of the cholesterol will be present in the buoyant, HDL2 fraction in contrast to individuals with a lower HDL-C level where more HDL cholesterol is shifted into the denser, HDL3 fraction.27 Such an effect is also observed with some synthetic CETP inhibitors such as torcetrapib which also preferentially increase the HDL2 cholesterol level, but through a different mechanism of action.28
The objective of this investigation was to determine what properties differentiate apoptotic and non-apoptotic HDL subclasses in adults with and without CHD. Our results suggest that the apoptotic HDL subclass described herein appears to be similar to the apoC-I-enriched HDL originally described by Kolmakova et al.20 and by Kwiterovich et al.21 in infant cord blood with the exception that it contains a higher molecular weight isoform of apoC-I. Characterization of this HDL phenotype is important in order to understand how HDL can function to promote atherosclerosis and how to identify individuals with this type of HDL who might otherwise be perceived to have a lower risk of CHD due to a generally higher HDL-C level.
2. Methods
2.1. Subjects
This investigation was approved by the Scott & White Institutional Review Board and informed consent was obtained from all subjects. The investigation conforms to the principles outlined in the Declaration of Helsinki29 for human subjects. The index case which led to this investigation was an individual with CHD who was found to have an HDL phenotype nearly identical to that described by Kwiterovich et al.21 Using an existing serum library of 200 individuals with and without CHD, we have thus far identified nine additional individuals with definitive evidence of this HDL phenotype. Samples were screened based on the presence of a prominent HDL2 particle distribution. The characteristics of each participant are summarized in Table 1. Serum was obtained after a 10–12 h fast and stored without preservative at −80°C. Eight subjects had angiographically proven CHD and a history of either percutaneous intervention and/or coronary artery bypass surgery. Another participant had presumed CHD based upon the presence of a left bundle branch block on their electrocardiogram (ECG) and the presence of cerebrovascular and peripheral arterial disease, both considered CHD risk equivalent diseases by the National Cholesterol Education Program (NCEP), Adult Treatment Panel III guidelines.30 Thus, a total of nine subjects had CHD or CHD risk equivalent disease. A group of eight individuals who had a normal coronary angiogram (no lesions >10%) within the 6 months prior to enrolment comprised the control group. These individuals had no history of symptomatic cerebrovascular disease, peripheral arterial disease, or other CHD risk equivalent disease. Also included were four subjects who had a high HDL-C level (>60 mg/dL) and a prominent HDL2 distribution, but no clinical history of CHD. Each of these four subjects had some objective evidence for the absence of obstructive CHD (e.g. a normal stress test, ECG, and/or echocardiogram) and no history of symptomatic cardiovascular disease (CVD) or CHD risk equivalent disease, but they did not have coronary angiography. All but 1 of the 21 subjects had at least one classic CHD risk factor (as defined by the NCEP30). Statin therapy was prescribed in all of the subjects with CHD and in about half of the subjects without CHD. Experiments evaluating the stability of the subclass distribution and stability of the in vitro properties were performed using a second serum sample from eight subjects with apoptotic HDL and a third blood draw from one these eight subjects.
Table 1.
Subject characteristics, lipid, apolipoprotein, and CETP activity*
| Subject | Age (years/race gender) | Angio. or other testing | Statin Rx | HTN | FHx | Tobacco | HDL-C | LDL-C | TC | TG | Lp(a) | C-I | A-I | A-II | C-III | E | B-100 | CETP activity |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 48/WF | Nl | Y | Y | 62 | 74 | 155 | 94 | 6.3 | 157.5 | ||||||||
| 3 | 77/WF | Nl | N | N | Y | 71 | 130 | 226 | 124 | 8.6 | 163.0 | |||||||
| 7 | 44/WF | Nl | N | N | Y | 76 | 95 | 185 | 70 | 5.3 | 170.8 | |||||||
| 10 | 79/WF | MVD | Y (estrogen) | N | Y | 71 | 84 | 173 | 92 | 103 | 13.3 | 153.8 | 42.6 | 11.4 | 7.6 | 74.0 | 20.0 | |
| 13 | 66/HF | Nl | N | Y | 51 | 64 | 130 | 77 | 7.4 | 165.0 | ||||||||
| 14 | 76/WF | Nl | Y | N | Y | F | 89 | 115 | 220 | 81 | 10.9 | 152.0 | ||||||
| 16 | 74/WF | Nl | Y | N | 42 | 145 | 223 | 179 | 7.1 | 138.0 | ||||||||
| 24 | 78/WF | Nl | N | Y | Y | F | 50 | 109 | 50 | 225 | 10.2 | 138.8 | ||||||
| 25 | 50/BM | Nl | Y (ezetimibe) | Y | F | 64 | 75 | 64 | 160 | 7.5 | 169.0 | |||||||
| 41 | 74/WM | MVD | Y | Y | F | 59 | 87 | 166 | 102 | 125.0 | 11.6 | 148.6 | 42.4 | 9.9 | 7.1 | 77.4 | 20.7 | |
| 47 | 72/WF | CHD equiv | N (estrogen) | Y | 94 | 135 | 255 | 129 | 7.0 | 26.4 | 168.8 | 6.2 | ||||||
| 49 | 56/WF | a | N (estrogen) | N | Y | 76 | 140 | 231 | 75 | 9.0 | 16.5 | 155.1 | 46.7 | 11.6 | 6.2 | 94.0 | 31.0 | |
| 79 | 40/WF | a | N | N | Y | F | 88 | 160 | 144 | 277 | 115 | 11.1 | 176.8 | 54.6 | 12.0 | 3.9 | 103.1 | 46.8 |
| 84 | 37/WF | MVD | Y | Y | Y | C | 43 | 36 | 110 | 154 | 39.0 | 7.9 | 134.3 | 32.6 | 12.2 | 2.6 | 67.7 | 13.7 |
| 129 | 82/WF | b | N | N | 61 | 72 | 166 | 165 | 32.0 | 10.6 | 151.1 | 38.4 | 11.5 | 4.4 | 84.2 | 17.8 | ||
| 141 | 59/WF | MVD | Y (niacin) | N | C | 41 | 76 | 156 | 193 | 77.6 | ||||||||
| 143 | 54/WM | MVD | Y | Y | F | 70 | 72 | 151 | 45 | 227 | 10.6 | 148.5 | 37.8 | 9.0 | 1.7 | 63.4 | 28.3 | |
| 146 | 77/WF | MVD | Y (estrogen) | Y | Y | C | 86 | 85 | 189 | 92 | 14.4 | 13.6 | 162.5 | 42.9 | 12.0 | 4.3 | 82.6 | 39.9 |
| 170 | 75/WM | MVD | Y (niacin) | Y | 91 | 61 | 163 | 56 | 29.3 | 16.5 | 158.4 | 35.8 | 11.9 | 3.7 | 55.9 | 22.8 | ||
| 193 | 61/WF | a | Y | N | 85 | 70 | 165 | 52 | 46.5 | 13.9 | 165.2 | 36.6 | 9.0 | 2.6 | 74.8 | 32.7 | ||
| 195 | 72/WF | MVD | Y | Y | 102 | 82 | 206 | 110 | 13.5 | 15.5 | 166.9 | 49.8 | 13.6 | 5.8 | 91.8 | 6.9 |
*All lipid and apolipoprotein values specified as mg/dL. Lp(a)=lipoprotein-a, CETP activity is in pmol/uL/h. The gray shading indicates a subject with a normal coronary angiogram and no CHD risk equivalent disease.
Abbreviations (i) for race and gender: W, white; B, black; H, hispanic; F, female; M, male. (ii) For angiography: Nl, normal coronary angiogram (i.e. no lesions showing >10% obstruction); MVD, multivessel disease; CHD equiv, CHD risk equivalent. (iii) For statin Rx: Y, yes; N, no; other notable lipid-altering medications are as specified. (iv) Risk factors: HTN, hypertension; FHx, positive family history of CVD at any age; tobacco use was characterized as C, current, or F, former (> 1 month smoke free); if not noted, there was no history of tobacco use. Only one subject (#84) had diabetes mellitus.
aNormal stress test.
bNormal ECG and no symptomatic CHD or CHD risk equivalent.
2.2. Apolipoprotein, lipid, and CETP
ApoA-I and apoC-I were measured in the Lipid and Lipoprotein Laboratory at the Oklahoma Medical Research Foundation (OMRF) for 20 of the 21 subjects; other apolipoproteins including A-II, B100, C-III, and E were measured in 12 subjects having sufficient serum for the assays. Lipid (n = 21) and lipoprotein-a levels (n = 13) were analysed by standard methods in the Clinical Chemistry Laboratory at Scott & White. CETP activity was measured in a subset of subjects (n = 11) with apoptotic HDL2 by Roar Biomedical Inc. (New York, NY, USA) utilizing a commercial assay (RB-CETP).
2.3. Lipoprotein subclass characterization and isolation
The lipoprotein subclasses were characterized and isolated by density gradient ultracentrifugation (DGU) using a metal-ion ethylene diamine tetraacetic acid (EDTA) density-forming solute for every sample.31 For the experiments described below, 0.18 M sodium bismuth EDTA (NaBiEDTA) (TCI America, Portland OR, USA) was used to characterize the overall lipid profile and to identify individuals with a prominent distribution of more buoyant HDL2 particles while 0.3 M dicesium cadmium EDTA (Cs2CdEDTA) synthesized by a previously published procedure32 was used to isolate the HDL subclasses. The latter solute provides superior resolution of the HDL2 and HDL3 subclasses as well as a better separation of HDL2 from the LDL and lipoprotein-a particles, but it does not resolve the LDL and VLDL particle distributions. The lipoprotein density distribution was imaged and converted into a spectral pattern using the fluorescence signal from the lipophilic NBD C6-ceramide (6-((N-(7-nitrobenz-2-oxa-1,3-diazol-4-yl)amino)hexanoyl)sphingosine) (Invitrogen, Carlsbad, CA, USA). The incorporation of NBD C6-ceramide was proportional to the cholesterol content of the lipoprotein particle. There was a strong linear relationship between the fluorescence intensity and the lipoprotein particle cholesterol concentration with an R2 value of 0.96.
2.4. Matrix-assisted laser desorption ionization-time-of-flight mass spectrometry
HDL fractions were desalted and delipidated as previously described.33 Mass spectral data of the HDL2 and HDL3 fractions were obtained using an Applied Biosystems Voyager-DE STR (Foster City, CA, USA) system for 16 participants: 7 subjects with CHD, 1 participant without CHD but with a markedly apoptotic effect of HDL on ASMCs, and all 8 subjects without angiographic evidence of CHD.
2.5. Fluorescence microscopic-quantitative assay of ASMC apoptosis and caspase-3 immunostaining
The same procedure described by Kolmakova et al.20 was used to measure the effect of HDL on ASMC apoptosis for all 21 subjects. In brief, 103 human ASMCs (Cambrex, Walkersville, MD, USA) were grown on sterilized glass cover slips in six-well trays and treated with 2.5 µL of the various lipoprotein fractions per mL of medium, 20 ng/mL tumour necrosis factor-α (TNF-α) (Sigma Aldrich, St Louis, MO, USA), 10 ng/mL C2-ceramide (Matreya, Pleasant Gap, PA, USA), or the cell medium alone. After a 24 h incubation, the medium was removed and cells were fixed and stained. The average apoA-I concentration in the HDL2 and HDL3 fractions was ∼40 and ∼25 mg/dL, respectively. The number of apoptotic cells was determined by fluorescence microscopic quantitative analysis using 4′,6-diamidino-2-phenylindole (DAPI) (Santa Cruz Biotechnology, Santa Cruz, CA, USA) staining. Approximately 300–500 cells in each sample were counted and the percent of apoptotic cells was plotted. Kolmakova et al.20 previously established a good correlation between this measurement of apoptosis with DNA laddering in their original description of ASMC apoptosis by HDL. The morphology of the ASMCs, characterized by light microscopy and α-smooth muscle cell actin (data not shown), demonstrated that the cultures were proliferating smooth muscle cells consisting of both contractile (small spindle-shaped cells) and long-non-contractile (relaxed) type of smooth muscle cells. All experiments were conducted within passages 3–6 of the cells.
The extent of ASMC apoptosis was confirmed by immunohistochemical staining using a caspase-3 antibody as previously described by Kolmakova et al.20 In six-well trays, human ASMCs (Cambrex, Walkersville, MD, USA) were cultured on sterilized glass cover slips as control (serum-free medium), 2.5 µL of the lipoprotein fractions per mL of serum free medium was added, and cells were incubated for 24 h in 5% CO2 followed by washing in phosphate-buffered saline (PBS) for 5 min/wash. ASMCs were fixed in 4% formalin for 24 h, washed, and then permeabilized with 0.5% Triton X-100 in PBS for 15 min. Cells were incubated with a primary antibody to caspase-3 (rabbit polyclonal IgG, Santa Cruz Biotechnology, Santa Cruz, CA, USA) at a 1:200 dilution overnight at 4°C. Next, ASMCs were blocked in 5% bovine serum albumin in PBS for 1 h at room temperature, washed, and incubated with a secondary antibody (goat anti-rabbit, tagged with Alexa-Fluor-488, Invitrogen, Eugene, OR, USA) for 1 h at room temperature, and washed. ASMC cover slips were mounted and imaged by confocal microscopy (LSM-meta 510 Zeiss, Jena, Germany) at ×40 oil magnification. The caspase-3 fluorescence was quantified using the ImageJ software (download-imagej.com).
2.6. Statistical analyses
Correlations were examined using the Pearson correlation coefficient. Apoptosis assays were performed in triplicate. Values were expressed as mean ± SD. The Student's t-test was used to evaluate the statistical significance of data. P < 05 was considered as significant.
3. Results
3.1. Apolipoprotein, lipid, and CETP activity levels
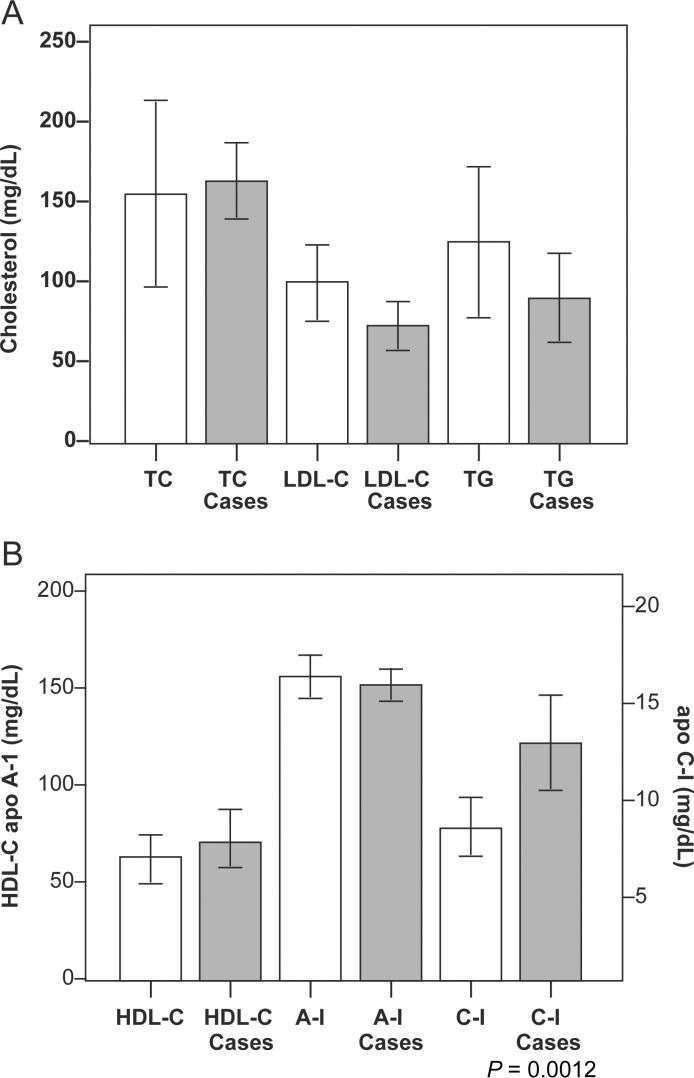
Lipid, apolipoproteins, and CETP activity levels for the subjects are summarized in Table 1. Differences in the lipid levels, apoC-I, and apoA-I between subjects with apoptotic HDL (cases) and subjects who had angiographically normal coronary arteries (controls) are summarized in Figure 1. The only significant difference was a notably higher mean apoC-I level in the subjects with apoptotic HDL compared with the controls (14.4 ± 5.64 vs. 7.9 ± 1.0 mg/dL, P = 0.0012), while apoA-I levels were nearly identical (155.2 ± 11.4 vs. 156.8 ± 12.8 mg/dL, P > 0.05). There was a strong, positive correlation of apoC-I with HDL-C (r = 0.72, P = 0.002). Among the eight subjects with CHD, this correlation was more robust (r = 0.96, P = 0.0006) and was equal to the correlation of HDL-C with apoA-I. The correlation with HDL-C was not improved by using the ratio of apoC-I/apoA-I or the ratio of apoC-I/(apoA-I + apoA-II). No other correlations between apolipoprotein values and HDL-C were statistically significant with P < 0.05. The mean CETP activity, measured in 11 subjects (4 without CHD), was 25.4 ± 11.4 pmol/µL/h. For the seven subjects with CHD, CETP activity was slightly lower with a mean value of 18.6 ± 6.8 pmol/µL/h, although this difference was not statistically significant.
Figure 1.
Mean value and standard deviation (error bars) for total cholesterol (TC), LDL-C, and triglyceride (TG) levels are shown in (A) and for HDL-C, apoA-I (values on left axis), and apoC-I (value on right axis) for serum from subjects with non-apoptotic (white bars, controls) and apoptotic (hashed bars, cases) HDL2 in (B). The only statistically significant difference between these two groups was a higher apoC-I concentration in subjects with apoptotic HDL2.
3.2. Lipoprotein subclass analyses
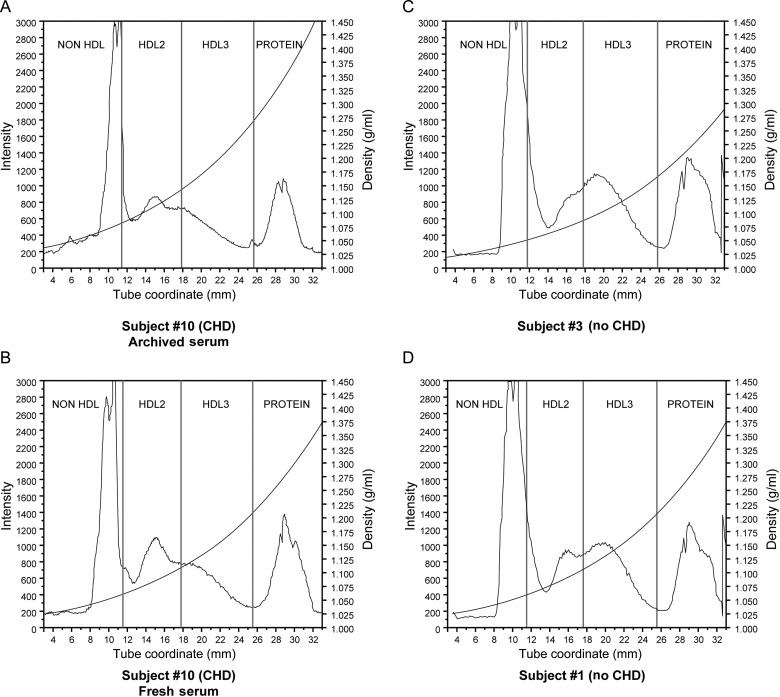
Using HDL fractions isolated by Cs2CdEDTA DGU, we observed the presence of a prominent distribution of HDL2 particles (as seen for participant #10 in Figure 2A) was generally associated with an increased stimulation of ASMC apoptosis, especially when the HDL-C level was high (>60 mg/dL). We also investigated the stability of the subclass properties over time using a second blood draw for five subjects with CHD and three subjects without CHD. The subjects had no significant changes in their medications or medical histories during the interval time ranging from 2 to 8 years. Although there were some minor changes in lipid levels, the lipoprotein density profiles and the extent of ASMC apoptosis (as described below) were unchanged. The comparison DGU profiles for participant #10 are shown in Figure 2A (initial blood draw) and B (second blood draw). A more prominent HDL3 subclass is typically observed for most of the subjects without apoptotic HDL as demonstrated by the DGU profile for a participant without CHD (subject #3) shown in Figure 2C. An exception was subject #1, where the profile showed almost equal distributions of the HDL2 and HDL3 subclasses as shown in Figure 2D.
Figure 2.
Using 60 µL of serum in 0.3 M Cs2Cd(EDTA), DGU spectra were obtained from (A) subject #10 (with CHD) using archived serum, (B) subject #10 using fresh serum, (C) subject #3 (no CHD), and (D) subject #1 (no CHD). The fluorescence intensity from NBD C6-ceramide is measured on the left axis. The vertical lines represent the cut points used to excise the HDL2 and HDL3 fractions. The curved line is the solute density corresponding to the values on the right axis.
The cut points for the HDL2 fraction were defined as d = 1.06–1.11 g/mL, while HDL3 fraction was defined as d = 1.11– 1.18 g/mL. These cut points are delineated by the vertical blue lines in the DGU spectra shown in Figure 2. Although there are slight differences in the lipoprotein particle density distributions between subjects, we used these constant and predetermined cut points to excise the HDL2 and HDL3 fractions rather than isolating fractions based on points of inflection in the DGU profile. This approach may be responsible for some of the variability we observed with respect to the extent of ASMC apoptosis (described below). Validity of the subclass distribution by the DGU method was previously assessed in a head-to-head comparison of DGU and classical gel electrophoresis reported in the original description of apoC-I-enriched HDL by Kwiterovich et al.21
3.3. Matrix-assisted laser desorption ionization-time-of-flight mass spectrometry
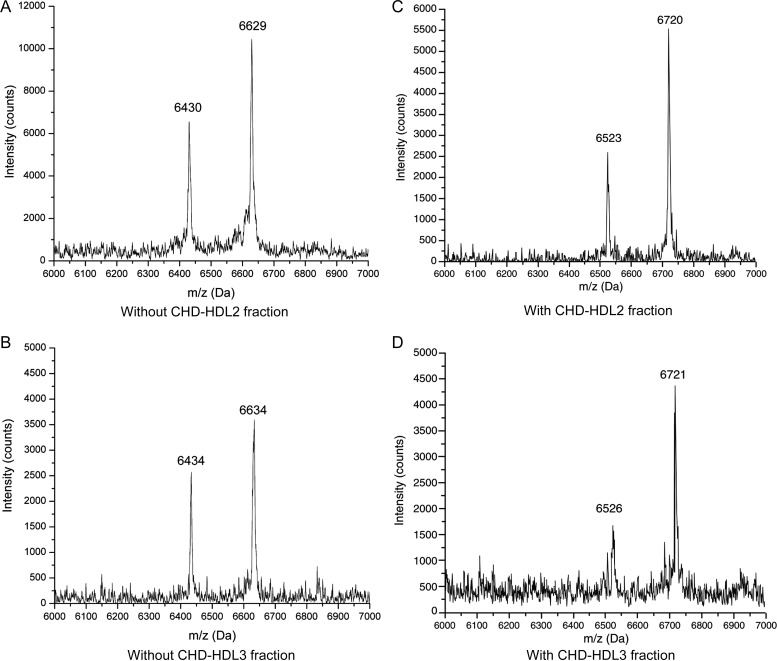
ApoC-I is present in humans34–36 and in great apes37 as the mature 57-amino acid protein (m/z 6630.6) and the post-translationally modified 55-amino acid protein (m/z 6432.5) lacking threonine and proline residues at the N-terminus. This doublet confers an immutable mass spectrometric signature, conserved across species. All subjects with a normal coronary angiogram exhibited this expected mass spectral pattern shown in Figure 3A and B corresponding to the full-length protein with an average m/z 6632.52 ± 1.98 and the truncated form, designated as apoC-I′ (apoC-I minus N-terminus Thr-Pro) with an average m/z 6432.95 ± 8.23. An unexpected finding we recently reported38 was a pronounced shift of this characteristic apoC-I/apoC-I′ doublet signature to a higher molecular weight isoform as seen in Figure 3C and D. The presence of this higher molecular weight apoC-I/apoC-I′ doublet, shifted by +90.98 ± 8.10 Da, was always associated with an apoptotic effect on ASMCs with the exception of subject #1 (a control without CHD) where HDL2 fraction induced apoptosis in 23% of the ASMCs, yet the mass spectrum demonstrated a normal apoC-I/apoC-I′ signature. Mass spectra of apoC-I/apoC-I′ in the HDL3 fractions, shown in Figure 3B and D, exhibited an identical apoC-I/apoC-I′ pattern as the HDL2 fraction but the peak intensities were lower. To prove this new isoform was a stable characteristic and not a result of sample aging, mass spectra were obtained of the delipidated HDL fractions isolated from the second and third blood draws (from subjects with apoptotic HDL). Each sample contained the higher molecular weight apoC-I isoform and no evidence of the normal apoC-I variant.
Figure 3.
MALDI mass spectra of the delipidated HDL2 (A and C) and HDL3 (B and D) fractions in the m/z 6000–7000 range. The doublet in all four mass spectra corresponds to the mature apoC-I apolipoprotein and the lower molecular weight apoC-I′ apolipoprotein associated with the loss of the N-terminus threonine and proline residues. (A) Delipidated HDL2 from subject #14 (normal angiogram, no evidence of HDL2-induced apoptosis); (B) delipidated HDL3 from subject #14; (C) delipidated HDL2 from subject #41 (multivessel coronary artery disease, apoptotic HDL2); (D) delipidated HDL3 from subject #41.
3.4. HDL-induced apoptosis in human ASMCs
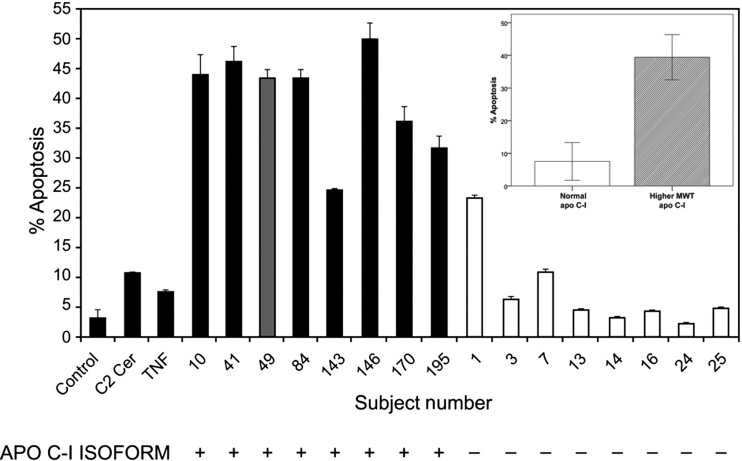
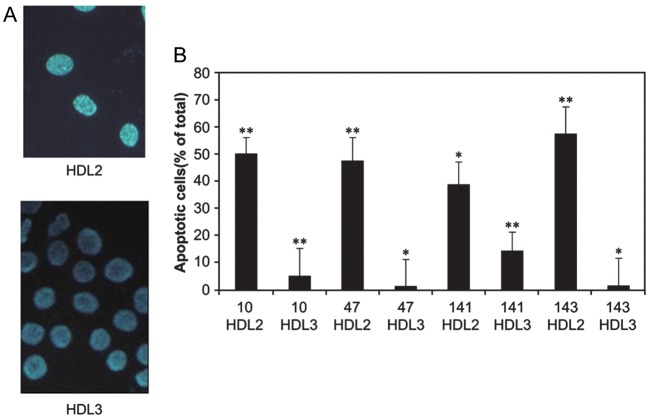
The effect of the HDL fractions on ASMC apoptosis is shown in Figures 4 and 5 for 18 of the 21 subjects. We observed that HDL2 isolated from individuals with CHD and one individual without CHD (subject # 49) induced up to ∼16-fold enhanced apoptosis compared with cells incubated with the cell medium (control). Figure 4 also demonstrates that the higher molecular weight isoform of apoC-I (noted by a ‘+’ sign) was detected in all of these cases and none of the non-apoptotic control samples and that the observed effect of HDL2 on ASMC apoptosis was up to 5–6-fold greater than C2-ceramide or TNF-α. Figure 5 shows that the apoptotic effect of HDL was primarily induced by the HDL2 fraction and not the HDL3 fraction from the same subject. In many respects, the HDL3 subclass serves as an internal control which reassures us that the apoptotic affect is influenced primarily, if not exclusively by the HDL particle composition and not extraneous experimental factors.
Figure 4.
Effect of HDL 2 from various subjects on ASMC apoptosis for subjects with CHD (represented by the black bars), a participant without known CHD (dark grey bar) and subjects with a normal coronary angiogram and no CHD risk equivalent disease (clear bars). The striking association of the higher-molecular weight apoC-I isoform in participants with ASMC apoptosis (represented by ‘+’ under each bar) compared with the subjects with a normal coronary angiogram and the normal apoC-I variant (represented by ‘–’ under each bar) is also demonstrated. The extent of apoptosis induced by HDL2 isolated from subjects with the higher molecular weight isoform of apoC-I was generally much higher than that induced by HDL2 isolated from subjects with the normal apoC-I variant as shown in the inset of Figure 4. With the exception of subjects #1and #7, those with the normal apoC-I variant did not induce significant ASMC apoptosis. There were no characteristics of these two controls (i.e. higher apoC-I or lower apoA-I/apoC-I levels, race, gender, risk factor burden, or medications) which would distinguish them from the other controls except for the observation that subject #1 had a more prominent HDL2 distribution as shown in Figure 1. Error bars represent the standard deviation of the mean cell counts performed in replicate measurements. Serum from subjects #10 and #143 was obtained using fresh serum.
Figure 5.
Effect of HDL2 and HDL3 from four subjects on ASMC apoptosis (right panel, B). Human ASMSs grown on glass cover slips were incubated with 2.5 µL of each fraction. The cells were stained with DAPI reagent. The nucleus of normal cells is blue and the nucleus of apoptotic cells is fragmented white as shown for most of the ASMCs shown in top micrograph (left panel, A) which were exposed to the HDL2 fraction from participant #10. This is in contrast to the nearly complete absence in any apoptotic cells in the bottom micrograph when the ASMCs were exposed to the HDL3 fraction. Error bars represent the standard deviation. The asterisks represent P values as follows: *P ≤ 0.05 , **P ≤ 0.005. Serum from subjects #10 and #143 were obtained using the first (older) serum samples. Error bars represent the standard deviation of the mean cell counts performed in replicate measurements.
We verified that the apoptotic property of HDL was a stable and reproducible characteristic over time by repeating the experiments using fresh serum samples. Comparing the results for subjects #10 and #143 in Figures 4 and 5 reveals more apoptosis in some cases (i.e. subject #10) but less in others (i.e. subject #143). Overall, we concluded the effect of the sample age was negligible. We believe the variability with respect to apoptosis was primarily affected by slight changes in the HDL particle distribution as seen in the DGU profiles in Figure 2. We also ruled out the possibility that the apoptotic effect was due to contamination of the HDL2 fraction by the higher-density apoB-containing lipoproteins, e.g. dense LDL and/or lipoprotein-a by: (i) incubating ASMCs with HDL2 containing the apoB fraction and (ii) removing the apoB-containing lipoproteins by precipitation prior to exposing the ASMCs to the HDL2 and HDL3 fractions. Neither of these actions affected the extent of HDL apoptosis. Incubation of cells with the non-HDL fraction (i.e. the apoB-containing lipoproteins) did not induce ASMC apoptosis (data not shown).
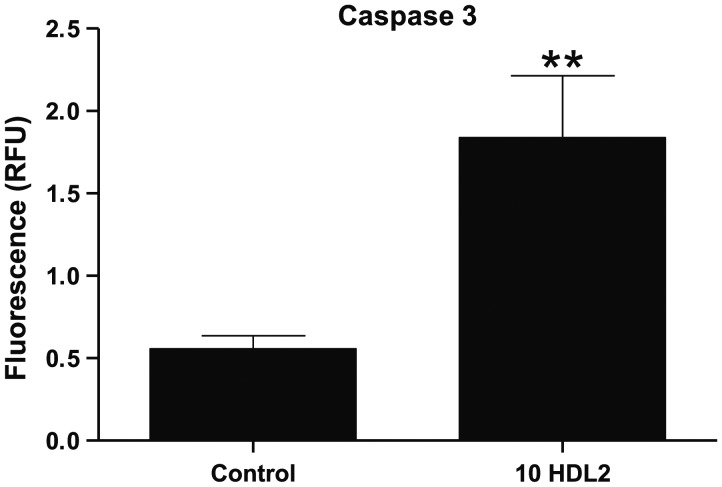
Kolmakova et al.20 found that expression of caspase-3, a crucial executioner in the pathway leading to DNA fragmentation and apoptosis, was stimulated by pure apoC-I and by apoC-1-enriched HDL but not by apoC-I-poor HDL. Thus, we conducted additional experiments to confirm such observations in this study and also as a second measure of the apoptotic effect of HDL2 fractions containing the higher molecular weight apoC-I isoform. An example shown in Figure 6 demonstrates that incubation of ASMCs with the HDL2 fraction from one such subject (#10) rendered a ∼3.5-fold increase in caspase-3 antibody fluorescence compared with HDL2 from a control subject with normal (non-apoptotic) HDL. This finding supports the extensive apoptosis observed by the cell morphology data using DAPI staining and is greater than the magnitude of the effect observed by Kolmakova et al.20
Figure 6.
Arterial smooth muscle cells were incubated with HDL2 from subject #10 (44.0% apoptosis by DAPI staining) and from a control, subject #14 (3.3% apoptosis by DAPI staining) for 24 h. Error bars represent the standard deviation of the mean cell counts for each sample. Note that cells incubated with HDL2 containing the modified apoC-I isoform reacted strongly to the capsase-3 antibody compared with HDL2 from a subject with normal apoC-I. This difference was statistically significant (**P = 0.0037). RFU = relative fluorescence units.
4. Discussion
Knowledge of the effects of HDL on cultured vascular cells provides insight into how HDL functions in circulation. Previous studies on cultured endothelial11–14 and smooth muscle cells15–17 have shown that HDL has a number of anti-atherogenic properties. In contrast, we have discovered a HDL subclass in adults which is associated with a profound apoptotic effect on human ASMCs in vitro, a property which could promote atherosclerotic plaque rupture, leading to thrombosis and myocardial infarction.21,23 The apoptotic effect was strongly associated with the presence of a higher molecular weight apoC-I isoform in the HDL particle, as well as generally higher apoC-I and HDL-C levels and a predominance of more buoyant HDL2 particles. To our knowledge, the promotion of ASMC apoptosis is one of the most potentially proatherogenic effects of HDL reported in the adult population to date.
This study confirms and extends the previous observations by Kolmakova et al.20 who first demonstrated that HDL isolated from LBW infants with an increased level of large HDL particles enriched in apoC-I could markedly induce ASMC apoptosis compared with apoC-I -poor HDL or pure apoC-I. Kwiterovich et al.21 hypothesized that if this HDL phenotype were to persist into adulthood, it could be a risk factor for CVD and explain the association between LBW and adult CVD originally described by Barker et al.39 Our data show that a nearly identical HDL phenotype is present in adults except it is associated with a higher molecular weight apoC-I mutation. This isoform of apoC-I was not observed in the matrix-assisted laser desorption ionization mass spectra of apoptotic apoC-I-enriched HDL from the LBW infants; but in that case, apoptosis was evaluated using pooled cord blood.21 Therefore, a direct correspondence with the apoC-I mass and apoptosis was not established as we have done in the experiments reported herein. Kwiterovich et al.21 reported the mass spectrum for only 1 of the 30 infants with the apoC-I-enriched HDL phenotype thus, the higher molecular weight apoC-I isoform may have been present in cord blood, but not detected. Although one participant with apoptotic HDL was aware they were ‘small and premature’, none of the other subjects knew any details of their birth history or their birth weight.
We did not find a statistically significant correlation with the total apoC-I level (or the apoA-I/apoC-I ratio) and the extent of ASMC apoptosis; this experimental aspect could not be addressed in the prior experiments by Kolmakova et al.20 because a pooled cord blood specimen was used. We believe that the absence of a correlation between the apoC-I concentration and apoptosis could be due to several factors. One is that the concentration in serum may not reflect the apoC-I enrichment in the HDL2 particle and/or levels of the higher molecular weight isoform which is closely associated with the apoptotic property of HDL2. Another explanation is the possibility that the apoptotic effect may not be due to the apoC-I isoform but rather a component closely correlated with the presence of the apoC-I isoform. The extent of apoptosis by ASMC morphology was confirmed by observing robust caspase-3 expression. This method correlates well with the TUNEL assay and has been shown to be a reliable indicator of apoptosis.40 We observed a ∼3.5-fold enhanced caspase-3 expression for HDL2 fractions that stimulated apoptosis by DAPI staining compared with HDL2 from subjects with non-apoptotic HDL2, whereas Kolmakova et al.20 reported a 1.7-fold enhancement by apoC-I-enriched HDL compared with the cell medium, and no enhancement for apoC-I-poor HDL.
Multiple apoC-I polymorphisms have been described in the literature, but only one other structural isoform of apoC-I has been reported in humans. The T45S variant, which affects adipocyte regulation in aboriginal Canadians36 and in persons of American Indian or Mexican ancestry35 results in an additional protein product 14 Da lower than the normal C-I variant. To our knowledge, higher molecular weight variants of apoC-I have not been reported in humans. Although apoC-I is synthesized containing an additional 26 unit peptide, it is removed prior to secretion and no evidence of a precursor propeptide or a propeptide fragment has ever been detected in circulation. The complete absence of the normal apoC-I variant suggests this isoform is not a post-translational modification given such modifications result in a mixture of products (normal and modified). An alternate explanation we considered is that the higher molecular weight isoform is the result of a genetic mutation. Puppione et al.37 have discovered a pseudogene product corresponding to a higher molecular weight form of apoC-I product shifted by +58 Da in great apes. Although there is significant homology between human and great ape genes, the human pseudogene, located downstream from the apoC-I gene, contains an intervening stop codon in the protein coding sequence and no mRNA product has ever been detected.41,42 Thus, it is unlikely the higher molecular weight apoC-I isoform we observed is due to the human pseudogene product. Further experiments are in progress to identify the origin of the higher molecular weight isoform.
The physiological role of apoC-I is quite wide and varied. Most pertinent to our observations is that apoC-I accounts for most of the CETP-inhibitory action associated with HDL, but the mechanism of action is quite different from the CETP blockade by pharmacological inhibitors such as torcetrapib. Gautier et al.25 have shown that purified human apoC-I at concentrations above 2 µg/mL nearly abolishes CETP activity, and secondly that the inhibitory action of HDL on CETP is proportional to the C-I content. We observed that the CETP activity, measured in a subset of 11 subjects in this study with apoptotic HDL, was a factor of approximately four times lower compared with values reported from other studies utilizing the same commercial assay. For example, in the Multi-Ethnic Study of Atherosclerosis, CETP activity ranged from 35.1 to 55.9 pmol/µL/h43 and in the Framingham Heart Study the mean CETP activity was 149 ± 6.8 85 pmol/µL/h.44 We did not find a significant correlation between the apoC-I concentration and CETP activity in either the cohort with apoptotic HDL or the controls with normal HDL, a finding also reported by Kwiterovich et al.21 Using a different CETP activity assay, Pillois et al.45 recently reported a weak, but statistically significant negative correlation between the apoC-I level and CETP activity in a cohort of 240 patients with CHD (r = 0.133, P < 0.001); the correlation improved to 0.330 (P < 0.001) in 101 normolipemic subjects within this cohort. Although they also observed a positive correlation with the apoC-I concentration and the HDL-C level, it was fairly weak (r = 0.165, P < 0.001) in the overall cohort and only slightly stronger in the normolipemic cohort (r = 0.330, P < 0.001). In contrast, the mean apoC-I concentration in the subjects reported herein was almost a factor of 2 higher than that of the cohort Pillois et al. studied, and we observed a much stronger, statistically significant positive correlation between the apoC-I concentration and the HDL-C level (r = 0.72–0.96).
Finally, another intriguing role for apoC-I which merits note is that higher levels also appear to confer a survival benefit in patients with endotoxemia by stimulating TNF-α production.46 Plomgaard and Nielsen47 have suggested that this proinflammatory effect of apoC-I, while beneficial in the setting of sepsis, may ultimately be detrimental because it could promote the future development of atherosclerosis through enhanced inflammation.
There are several aspects of our experimental approach that differ from other investigations of how HDL affects vascular cells. One is that we used a relatively new ultracentrifugation method to isolate the HDL subclasses. When lipoproteins are isolated by ultracentrifugation, there is some concern that the HDL particle properties may be influenced by protein shedding during centrifugation. The literature suggests that the in vitro functional properties of HDL subclasses isolated using conventional NaBr or KBr DGU12–14 are preserved. Compared with these methods, the DGU method utilizes a lower ionic strength gradient (∼0.6 M for 0.3 M Cs2Cd (EDTA), vs. ∼3 M for NaBr or KBr) and a reduced spin time (6 vs. 20–30 h) so the DGU method is less likely to perturb the HDL particle integrity. Reassuring evidence that the isolation method did not affect the in vitro properties of HDL is provided by noting that the extent of ASMC apoptosis produced by HDL2 and HDL3 isolated by DGU is nearly identical to that observed for apoC-I-enriched HDL and apoC-I-depleted HDL, respectively, isolated by sequential immunoprecipitation in the original description of this HDL phenotype.20
Another difference with our approach is that most in vitro studies have examined the effects of HDL on vascular cells using plasma. This includes Kolmakova's initial investigations of apoC-I-enriched HDL on ASMC.20 We have historically used serum since our initial experiments were performed using leftover serum from a standard lipid profile. Collins and Olivier48 determined that the only significant differences between the lipoprotein composition in plasma and in serum are primarily attributable to the absence of fibrinogen proteins as well as a slightly higher level of apoB-100 in serum compared with plasma. Thus, we believe the extent of ASMC apoptosis would be the same for HDL isolated from plasma.
We also acknowledge that a weakness of this study is the limited population diversity with respect to gender, ethnicity, and the cross-sectional design. The preponderance of women in this study was explained in part by our screening criteria which focused on subjects with a prominent HDL2 subclass distribution given this was the phenotypic signature of infants with apoC-I-enriched HDL independent of their gender.21 The circulating level of the HDL2 subclass is typically higher in adult women than men.49 The predominately female gender in our sample may have therefore biased the apparent association of apoC-I with higher HDL-C levels. Approximately 80% of subjects enrolled in the clinical studies supporting the serum library for this investigation were Caucasian. This in part reflects the demographics of the population but also the smaller number of minority populations agreeing to participate in studies.
In summary, our results demonstrate that some HDL2 subclasses containing a higher molecular weight isoform of apoC-I markedly enhance smooth muscle cell apoptosis in vitro. Our findings corroborate the observations of Kwiterovich et al.21 and Kolmakova et al.20 pointing to the existence of an HDL subclass which may find its origins in an unfavourable intrauterine environment. HDL containing this apoC-I isoform may be expected to unfavourably impact CHD outcomes based upon additional supporting in vivo evidence from Steen et al.22 demonstrating a compelling association of apoC-I with capsase-3 and ceramide at the site of atherosclerotic plaque rupture in a rabbit model and the proinflammatory effect of apoC-I reported by Plomgaard and Nielsen.47 Because individuals with this HDL subclass generally have higher HDL-C levels, likely resulting from the inhibitory effects of apoC-I on CETP, they may paradoxically be perceived to have a lower risk of CHD. This study therefore also offers an example of a mechanism associated with a potentially adverse effect of CETP inhibition on CHD risk.
We hypothesize that at the vascular level, this HDL subclass, with access to the subintimal space, may exert toxic effects on vascular cells which promote atherogenesis and contribute to plaque rupture based on our knowledge of the underpinning molecular mechanisms linking smooth muscle cell apoptosis and inflammation with enhanced plaque instability.23 Our findings further support a growing body of evidence demonstrating that measures of HDL function may be more informative with respect to the CHD risk than the HDL-C level. A failure to recognize this HDL subclass as a putative risk factor may result in an underestimation of an individual's risk for CHD, especially in those individuals having high HDL-C levels.
Funding
This work was supported by grants from Scott & White Healthcare (R3524); the Scott & White Department of Medicine (C.J.M.); the National Institutes of Health Heart, Lung and Blood Institute (RO1 HL068794) (R.D.M.) and National Institute of Health [1 P30DK090868, PO1-HL-107153-01 (S.C.)] and a TEDCO grant from the state of Maryland (S.C.).
Acknowledgements
We are grateful for the assistance of the following individuals: Carmen Quiroga who performed the apoplipoprotein analyses at the OMRF; from Texas A&M University, D'Vesharronne Moore and Paul Cammarata who helped obtain the mass spectral data, and Jeffrey Johnson who carried out some of the DGU separations; and Archana Murali, for assistance with the apoptosis measurements at Johns Hopkins School of Medicine. We are also grateful for the insight provided by Dr Donald Puppione and Dr Julian Whitelegge regarding the apoC-I gene product expression.
Conflict of interest: none declared.
References
- 1.Assmann G, Gotto AM., Jr HDL cholesterol and protective factors in atherosclerosis. Circulation. 2004;109:III8–III14. doi: 10.1161/01.CIR.0000131512.50667.46. [DOI] [PubMed] [Google Scholar]
- 2.Tall AR. Cholesterol efflux pathways and other potential mechanisms involved in the athero-protective effect of high density lipoproteins. J Intern Med. 2008;263:256–273. doi: 10.1111/j.1365-2796.2007.01898.x. doi:10.1111/j.1365-2796.2007.01898.x. [DOI] [PubMed] [Google Scholar]
- 3.Toth PP, Davidson MH. High-density lipoproteins: marker of cardiovascular risk and therapeutic target. J Clin Lipidol. 2010;4:359–364. doi: 10.1016/j.jacl.2010.08.002. doi:10.1016/j.jacl.2010.08.002. [DOI] [PubMed] [Google Scholar]
- 4.Movva R, Rader DJ. Laboratory assessment of HDL heterogeneity and function. Clin Chem. 2008;54:788–800. doi: 10.1373/clinchem.2007.101923. doi:10.1373/clinchem.2007.101923. [DOI] [PubMed] [Google Scholar]
- 5.Asztalos BF, Tani M, Schaefer EJ. Metabolic and functional relevance of HDL subspecies. Curr Opin Lipidol. 2011;22:176–185. doi: 10.1097/MOL.0b013e3283468061. doi:10.1097/MOL.0b013e3283468061. [DOI] [PubMed] [Google Scholar]
- 6.Rye KA, Barter PJ. Predictive value of different HDL particles for the protection against or risk of coronary heart disease. Biochim Biophys Acta. 2012;1821:473–480. doi: 10.1016/j.bbalip.2011.10.012. doi:10.1016/j.bbalip.2011.10.012. [DOI] [PubMed] [Google Scholar]
- 7.Francis GA. The complexity of HDL. Biochim Biophys Acta. 2010;1801:1286–1293. doi: 10.1016/j.bbalip.2010.08.009. doi:10.1016/j.bbalip.2010.08.009. [DOI] [PubMed] [Google Scholar]
- 8.Navab M, Reddy ST, Van Lenten BJ, Fogelman AM. HDL and cardiovascular disease: atherogenic and atheroprotective mechanisms. Nat Rev Cardiol. 2011;8:222–232. doi: 10.1038/nrcardio.2010.222. doi:10.1038/nrcardio.2010.222. [DOI] [PubMed] [Google Scholar]
- 9.Khera AV, Cuchel M, de la Llera-Moya M, Rodrigues A, Burke MF, Jafri K, et al. Cholesterol efflux capacity, high-density lipoprotein function, and atherosclerosis. N Engl J Med. 2011;364:127–135. doi: 10.1056/NEJMoa1001689. doi:10.1056/NEJMoa1001689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Calabresi L, Gomaraschi M, Franceschini G. High-density lipoprotein quantity or quality for cardiovascular prevention? Curr Pharm Des. 2010;16:1494–1503. doi: 10.2174/138161210791050960. doi:10.2174/138161210791050960. [DOI] [PubMed] [Google Scholar]
- 11.Mineo C, Deguchi H, Griffin JH, Shaul PW. Endothelial and antithrombotic actions of HDL. Circ Res. 2006;98:1352–1364. doi: 10.1161/01.RES.0000225982.01988.93. doi:10.1161/01.RES.0000225982.01988.93. [DOI] [PubMed] [Google Scholar]
- 12.Kontush A, Therond P, Zerrad A, Couturier M, Négre-Salvayre A, de Souza JA, et al. Preferential sphingosine-1-phosphate enrichment and sphingomyelin depletion are key features of small dense HDL3 particles: relevance to antiapoptotic and antioxidative activities. Arterioscler Thromb Vasc Biol. 2007;27:1843–1849. doi: 10.1161/ATVBAHA.107.145672. doi:10.1161/ATVBAHA.107.145672. [DOI] [PubMed] [Google Scholar]
- 13.Barter PJ, Baker PW, Rye KA. Effect of high-density lipoproteins on the expression of adhesion molecules in endothelial cells. Curr Opin Lipidol. 2002;13:285–288. doi: 10.1097/00041433-200206000-00008. doi:10.1097/00041433-200206000-00008. [DOI] [PubMed] [Google Scholar]
- 14.de Souza JA, Vindis C, Nègre-Salvayre A, Rye KA, Couturier M, Therond P, et al. Small, dense HDL 3 particles attenuate apoptosis in endothelial cells: pivotal role of apolipoprotein A-I. J Cell Mol Med. 2010;14:608–620. doi: 10.1111/j.1582-4934.2009.00713.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liisanantti MK, Savolainen MJ. Phosphatidylethanol in high density lipoproteins increases the vascular endothelial growth factor in smooth muscle cells. Atherosclerosis. 2005;180:263–269. doi: 10.1016/j.atherosclerosis.2004.12.041. doi:10.1016/j.atherosclerosis.2004.12.041. [DOI] [PubMed] [Google Scholar]
- 16.González-Díez M, Rodríguez C, Badimon L, Martínez-González J. Prostacyclin induction by high- density lipoprotein (HDL) in vascular smooth muscle cells depends on sphingosine 1-phosphate receptors: effect of simvastatin. Thromb Haemost. 2008;100:119–126. doi: 10.1160/TH07-11-0675. [DOI] [PubMed] [Google Scholar]
- 17.Francis GA, Tsujita M, Terry TL. Apolipoprotein AI efficiently binds to and mediates cholesterol and phospholipid efflux from human but not rat aortic smooth muscle cells. Biochemistry. 1999;38:16315–16322. doi: 10.1021/bi991742b. doi:10.1021/bi991742b. [DOI] [PubMed] [Google Scholar]
- 18.Terasaka N, Wang N, Yvan-Charvet L, Tall AR. High-density lipoprotein protects macrophages from oxidized low-density lipoprotein-induced apoptosis by promoting efflux of 7-ketocholesterol via ABCG1. Proc Natl Acad Sci USA. 2007;104:15093–15098. doi: 10.1073/pnas.0704602104. doi:10.1073/pnas.0704602104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yvan-Charvet L, Pagler TA, Seimon TA, Thorp E, Welch CL, Witztum JL, et al. ABCA1 and ABCG1 protect against oxidative stress-induced macrophage apoptosis during efferocytosis. Circ Res. 2010;106:1861–1869. doi: 10.1161/CIRCRESAHA.110.217281. doi:10.1161/CIRCRESAHA.110.217281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kolmakova A, Kwiterovich P, Virgil D, Alaupovic P, Knight-Gibson C, Martin SF, et al. Apolipoprotein C-I induces apoptosis in human aortic smooth muscle cells via recruiting neutral sphingomyelinase. Arterioscler Thromb Vasc Biol. 2004;24:264–269. doi: 10.1161/01.ATV.0000112036.72200.ac. doi:10.1161/01.ATV.0000112036.72200.ac. [DOI] [PubMed] [Google Scholar]
- 21.Kwiterovich PO, Jr, Cockrill SL, Virgil DG, Garrett ES, Otvos J, Knight-Gibson C, et al. A large high-density lipoprotein enriched in apolipoprotein C-I: a novel biochemical marker in infants of lower birth weight and younger gestational age. JAMA. 2005;293:1891–1899. doi: 10.1001/jama.293.15.1891. doi:10.1001/jama.293.15.1891. [DOI] [PubMed] [Google Scholar]
- 22.Steen H, Kolmakova A, Stuber M, Rodriguez ER, Gao F, Chatterjee S, et al. MRI visualized neo-intimal dissection and co-localization of novel apoptotic markers apolipoprotein C-1, ceramide and caspase-3 in a Watanabe hyperlipidemic rabbit model. Atherosclerosis. 2007;191:82–89. doi: 10.1016/j.atherosclerosis.2006.05.022. doi:10.1016/j.atherosclerosis.2006.05.022. [DOI] [PubMed] [Google Scholar]
- 23.Clarke MC, Figg N, Maguire JJ, Davenport AP, Goddard M, Littlewood TD, et al. Apoptosis of vascular smooth muscle cells induces features of plaque vulnerability in atherosclerosis. Nat Med. 2006;12:1075–1080. doi: 10.1038/nm1459. doi:10.1038/nm1459. [DOI] [PubMed] [Google Scholar]
- 24.Cohn JS, Tremblay M, Batal R, Jacques H, Veilleux L, Rodriguez C, et al. Plasma kinetics of VLDL and HDL apoC-I in normolipidemic and hypertriglyceridemic subjects. J Lipid Res. 2002;43:1680–1687. doi: 10.1194/jlr.m200055-jlr200. doi:10.1194/jlr.M200055-JLR200. [DOI] [PubMed] [Google Scholar]
- 25.Gautier T, Masson D, de Barros JP, Athias A, Gambert P, Aunis D, et al. Human apolipoprotein C-I accounts for the ability of plasma high density lipoproteins to inhibit the cholesteryl ester transfer protein activity. J Biol Chem. 2000;275:37504–37509. doi: 10.1074/jbc.M007210200. doi:10.1074/jbc.M007210200. [DOI] [PubMed] [Google Scholar]
- 26.Dumont L, Gautier T, de Barros JP, Laplanche H, Blache D, Ducoroy P, et al. Molecular mechanism of the blockade of plasma cholesteryl ester transfer protein by its physiological inhibitor apolipoprotein CI. J Biol Chem. 2005;280:38108–38116. doi: 10.1074/jbc.M504678200. doi:10.1074/jbc.M504678200. [DOI] [PubMed] [Google Scholar]
- 27.Demacker PN, Baadenhuysen H, Stuyt PM, Van 't Laar A. Studies on the relationship between the cholesterol content in total high density lipoprotein and its subfractions, HDL2 and HDL3 in normo- and hyperlipidemic subjects. Atherosclerosis. 1986;61:225–229. doi: 10.1016/0021-9150(86)90142-5. doi:10.1016/0021-9150(86)90142-5. [DOI] [PubMed] [Google Scholar]
- 28.Bellanger N, Julia Z, Villard EF, El Khoury P, Duchene E, Chapman MJ, et al. Functionality of postprandial larger HDL2 particles is enhanced following CETP inhibition therapy. Atherosclerosis. 2012;221:160–168. doi: 10.1016/j.atherosclerosis.2011.12.027. doi:10.1016/j.atherosclerosis.2011.12.027. [DOI] [PubMed] [Google Scholar]
- 29.World Medical Association Declaration of Helsinki. Recommendations guiding physicians in biomedical research involving human subjects. Cardiovasc Res. 1997;35:2–3. doi:10.1016/S0008-6363(97)00109-0. [PubMed] [Google Scholar]
- 30.Grundy SM, Cleeman JI, Merz CN, Brewer HB, Jr, Clark LT, Hunninghake DB, et al. Implications of recent clinical trials for the National Cholesterol Education Program Adult Treatment Panel III guidelines. Circulation. 2004;110:227–239. doi: 10.1161/01.CIR.0000133317.49796.0E. doi:10.1161/01.CIR.0000133317.49796.0E. [DOI] [PubMed] [Google Scholar]
- 31.Larner CD, Henriquez RR, Johnson JD, Macfarlane RD. Developing high performance lipoprotein density profiling for use in clinical studies relating to cardiovascular disease. Anal Chem. 2011;83:8524–8530. doi: 10.1021/ac2018124. doi:10.1021/ac2018124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Johnson JD, Bell NJ, Donahue EL, Macfarlane RD. Metal ion complexes of EDTA as solutes for density gradient ultracentrifugation: influence of metal ions. Anal Chem. 2005;77:7054–7061. doi: 10.1021/ac0509657. doi:10.1021/ac0509657. [DOI] [PubMed] [Google Scholar]
- 33.Watkins LK, Bondarenko PV, Barbacci DC, Song S, Cockrill SL, Russell DH, et al. Fast C18 solid-phase desalting/delipidation of the human serum apolipoproteins for matrix-assisted laser desorption ionization and electrospray ionization mass spectrometric analysis. J Chromatogr A. 1999;840:183–193. doi: 10.1016/s0021-9673(99)00205-8. doi:10.1016/S0021-9673(99)00205-8. [DOI] [PubMed] [Google Scholar]
- 34.Bondarenko PV, Farwig ZN, McNeal CJ, Macfarlane RD. MALDI- and ESI-MS of the HDL apolipoproteins; new isoforms of apo A-I, II. Int J Mass Spectrom. 2002;219:671–680. doi:10.1016/S1387-3806(02)00709-1. [Google Scholar]
- 35.Wroblewski MS, Wilson-Grady JT, Martinez MB, Kasthuri RS, McMillan KR, Flood-Urdangarin C, et al. A functional polymorphism of apolipoprotein C1 detected by mass spectrometry. FEBS J. 2006;273:4707–4715. doi: 10.1111/j.1742-4658.2006.05473.x. doi:10.1111/j.1742-4658.2006.05473.x. [DOI] [PubMed] [Google Scholar]
- 36.Lahiry P, Cao H, Ban MR, Pollex RL, Mamakeesick M, Zinman B, et al. APOC1 T45S polymorphism is associated with reduced obesity indices and lower plasma concentrations of leptin and apolipoprotein C-I in aboriginal Canadians. J Lipid Res. 2010;4:843–848. doi: 10.1194/jlr.P002014. doi:10.1194/jlr.P002014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Puppione DL, Ryan CM, Bassilian S, Souda P, Xiao X, Ryder OA, et al. Detection of two distinct forms of apoC-I in great apes. Comp Biochem Physiol Part D Genomics Proteomics. 2010;5:73–79. doi: 10.1016/j.cbd.2009.12.003. doi:10.1016/j.cbd.2009.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Moore D, McNeal C, Macfarlane R. Isoforms of apolipoprotein C-I associated with individuals with coronary artery disease. Biochem Biophys Res Commun. 2011;404:1034–1038. doi: 10.1016/j.bbrc.2010.12.105. doi:10.1016/j.bbrc.2010.12.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Barker DJ, Winter PD, Osmond C, Margetts B, Simmonds SJ. Weight in infancy and death from ischaemic heart disease. Lancet. 1989;2:577–580. doi: 10.1016/s0140-6736(89)90710-1. doi:10.1016/S0140-6736(89)90710-1. [DOI] [PubMed] [Google Scholar]
- 40.Duan WR, Garner DS, Williams SD, Funckes-Shippy CL, Spath IS, Blomme EA. Comparison of immunohistochemistry for activated caspase-3 and cleaved cytokeratin 18 with the TUNEL method for quantification of apoptosis in histological sections of PC-3 subcutaneous xenografts. J Pathol. 2003;199:221–228. doi: 10.1002/path.1289. doi:10.1002/path.1289. [DOI] [PubMed] [Google Scholar]
- 41.Lauer SJ, Walker D, Elshourbagy NA, Reardon CA, Levy-Wilson B, Taylor JM. Two copies of the human apolipoprotein C-I gene are linked closely to the apolipoprotein E gene. J Biol Chem. 1988;263:7277–7286. [PubMed] [Google Scholar]
- 42.Freitas EM, Gaudieri S, Zhang WJ, Kulski JK, van Bockxmeer FM, Christiansen FT, et al. Duplication and diversification of the apolipoprotein CI (APOCI) genomic segment in association with retroelements. J Mol Evol. 2000;50:391–396. doi: 10.1007/s002399910042. [DOI] [PubMed] [Google Scholar]
- 43.Tsai MY, Johnson C, Kao WH, Sharrett AR, Arends VL, Kronmal R, et al. Cholesteryl ester transfer protein genetic polymorphisms, HDL cholesterol, and subclinical cardiovascular disease in the Multi-Ethnic Study of Atherosclerosis. Atherosclerosis. 2008;200:359–367. doi: 10.1016/j.atherosclerosis.2007.12.038. doi:10.1016/j.atherosclerosis.2007.12.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vasan RS, Pencina MJ, Robins SJ, Zachariah JP, Kaur G, D'Agostino RB, et al. Association of circulating cholesteryl ester transfer protein activity with incidence of cardiovascular disease in the community. Circulation. 2009;120:2414–2420. doi: 10.1161/CIRCULATIONAHA.109.872705. doi:10.1161/CIRCULATIONAHA.109.872705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pillois X, Gautier T, Bouillet B, Pais de Barros JP, Jeannin A, Verges B, et al. Constitutive inhibition of plasma cholesteryl ester transfer protein (CETP) by apolipoprotein C1 is blunted in dyslipidemic patients with coronary artery disease. J Lipid Res. 2012;53:1200–1209. doi: 10.1194/jlr.M022988. doi:10.1194/jlr.M022988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Berbee JF, van der Hoogt CC, Kleemann R, Schippers EF, Kitchens RL, van Dissel JT, et al. Apoliproprotein CI stimulates the response to lipopolysaccharide and reduces mortality in gram-negative sepsis. FASEB J. 2006;20:2162–2164. doi: 10.1096/fj.05-5639fje. doi:10.1096/fj.05-5639fje. [DOI] [PubMed] [Google Scholar]
- 47.Plomgaard P, Nielsen LB. Lipid metabolism: new insight into the modulatory role of apolipoprotein C-I in inflammation. Curr Opin Lipidol. 2008;19:431–432. doi: 10.1097/MOL.0b013e328306f0dd. doi:10.1097/MOL.0b013e328306f0dd. [DOI] [PubMed] [Google Scholar]
- 48.Collins LA, Olivier M. Quantitative comparison of lipoprotein fractions derived from human plasma and serum by liquid chromatography-tandem mass spectrometry. Proteome Sci. 2010;8:42. doi: 10.1186/1477-5956-8-42. doi:10.1186/1477-5956-8-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Johnson JL, Slentz CA, Duscha BD, Samsa GP, McCartney JS, Houmard JA, et al. Gender and racial differences in lipoprotein subclass distributions: the STRRIDE study. Atherosclerosis. 2004;176:371–377. doi: 10.1016/j.atherosclerosis.2004.05.018. doi:10.1016/j.atherosclerosis.2004.05.018. [DOI] [PubMed] [Google Scholar]