Abstract
The adaptations of root morphology, physiology, and biochemistry to phosphorus supply have been characterized intensively. However, characterizing these adaptations at molecular level is largely neglected under field conditions. Here, two consecutive field experiments were carried out to investigate the agronomic traits and root traits of wheat (Triticum aestivum L.) at six P-fertilizer rates. Root samples were collected at flowering to investigate root dry weight, root length density, arbusular-mycorrhizal colonization rate, acid phosphatase activity in rhizosphere soil, and expression levels of genes encoding phosphate transporter, phosphatase, ribonucleases, and expansin. These root traits exhibited inducible, inhibitory, or combined responses to P deficiency, and the change point for responses to P supply was at or near the optimal P supply for maximum grain yield. This research improves the understanding of mechanisms of plant adaptation to soil P in intensive agriculture and provides useful information for optimizing P management based on the interactions between soil P dynamics and root processes.
Key words: Agronomic trait, phosphatase, phosphate-starvation response, phosphate transporter, phosphorus fertilizer, root morphology, Triticum aestivum L.
Introduction
Phosphorus is one of the most important macronutrients that significantly affect plant growth and metabolism. Although total P content in soils is high, P availability is usually the lowest of the macronutrients (Cordell et al., 2009), and therefore plants often encounter a scarcity of phosphate (Pi) in soils of both agricultural and natural systems (Raghothama, 2000). In intensive agriculture, large amounts of inorganic P fertilizers and organic manures are applied to overcome soil P deficiency. However, because rock phosphate is a non-renewable resource and most P applied to soil is immobilized and becomes unavailable for plants and the recovery of applied P by crops in one growing season is often low (Vance et al., 2003), improving the efficiency of P use is therefore important in sustainable agriculture.
Soil-based P management, which manipulates soil P according to yield response to P fertilizer, has been long and widely used in optimizing P use (Kirkby and Johnston, 2008). Considering the vital roles of rhizosphere processes in P use, scientists have developed approaches to modify rhizosphere processes in increasing the efficiency of P use. In response to low availability of P in the rhizosphere, plants have developed highly specialized morphological, physiological, and biochemical adaptive mechanisms to modify the rhizosphere and, hence, increase the ability of their root systems to utilize Pi from soils (Vance et al., 2003; Lynch and Brown, 2008; Hinsinger et al., 2009; Zhang et al., 2010; George et al., 2011). The conserved responses include: (1) investment of a greater proportion of photosynthates in the roots, alterations in root morphology, and establishment of symbiotic relations with arbusular-mycorrhizal (AM) fungi to increase exploration of the soil volume (Hermans et al., 2006; Bucher, 2007; Hammond and White, 2008; Lynch and Brown, 2008; Péret et al., 2011; Smith and Smith, 2011); (2) increased proton release and secretion of organic anions and phosphatase enzymes into the soil to mobilize Pi from inorganic and organic P sources in the rhizosphere (Marschner, 1995; Hinsinger, 2001; Jones et al., 2003; Jain et al., 2007; George and Richardson, 2008); and (3) enhancing the capacity of root cells to take up Pi by increasing the employment of high-affinity Pi transporters (Liu et al., 1998; Raghothama, 1999; Vance et al., 2003). Understanding these responses provides useful information to increase mobilization and acquisition of P by crops through both modifying rhizosphere processes and the so-called rhizosphere-based P management (Zhang et al, 2010; Li et al., 2011a). Rhizosphere-based P management aims to modify rhizosphere processes by localized supply of nutrients, intercropping (e.g. maize/faba bean intercropping), and exploitation of plant genetic potential through conventional and molecular breeding (Li et al., 2011a). For example, non-uniform supplies of nutrients have been long known to stimulate root branching (Robinson, 1994). A recent study in maize showed that localized application of P plus ammonium stimulated root branching and rhizosphere acidification and thus significantly increased P uptake, compared with broadcast application (Jing et al., 2010). Recent advances in marker-assisted selection and gene transformation have greatly increased the efficiency of breeding crops with improved root traits (Tian et al., 2012), which has been shown effective in improving P uptake by manipulating genes regulating root architecture (Guo et al., 2011; Li et al., 2011b), Pi mobilization from insoluble P pools in rhizosphere (Lopez-Bucio et al., 2000; Richardson et al., 2001; Wang et al., 2009; Liang et al., 2010), and Pi transportation (Seo et al., 2008; Ai et al., 2009).
As the ability of roots to utilize soil P is affected by soil P supply and rhizosphere processes, effective strategies for P management should involve multidisciplinary approaches based on the soil and the rhizosphere processes (Li et al., 2011a; Shen et al., 2011). The development of effective strategies requires better understanding of the root response to soil P supply in at least the following two aspects. First, estimation of the critical soil P concentration to trigger root responses and its relation with the optimal soil P level for crop production is needed. Previous studies showed that there is a critical P supply that triggers P-starvation response. For example, the transcripts of the P-starvation marker gene Mt4 in the roots of Medicago truncatula were abundant when the plants grown in nutrient solution containing no Pi but were reduced with 0.02 and 0.1mM Pi and became undetectable with 1 and 5mM Pi (Burleigh and Harrison, 1998). Hill et al. (2006) compared the root morphological parameters of various pasture species grown in pots fertilized with six P concentrations and found that root adaptations were triggered at or near the critical soil P supply for 90% of maximum shoot mass. In intensive agriculture, although optimal P supplies for crop production have been estimated worldwide in a number of crops (Kirkby and Johnston, 2008), estimation of the critical P supply that initializes the morphological, physiological and biochemical responses of underground roots is largely neglected. Second, a better understanding of the morphological, physiological, and biochemical properties of the roots grown in P deficient, optimal, and excessive soils is needed. Numerous publications have described the regulation of P supply on root traits at the morphological and physiological levels and, more recently, at the molecular level; however, the majority of the researches focused on short-term responses under controlled conditions, and on-farm field-scale and systematic research to characterize the long-term responses of roots to P supply from the morphological and physiological to the biochemical aspects is still lacking. Taking these two aspects into account will enable the prediction of root processes and will be useful for the development of effective management strategies targeting both high yield and efficient P use.
Wheat is one of the most important food crops in the world and consumes much more P fertilizer than rice (Oryza sativa L.) and maize, both in terms of annual total consumption and in terms of consumption per unit area (Food and Agriculture Organization, 2006). Therefore, improving the efficiency of P uptake in wheat is important to the sustainable use of P resources. This study investigates the responses of agronomic performance and root traits to P supply from deficiency to excess in two field experiments in the main wheat area of China. The results show that the optimal soil P supply for maximum yield is at or near the critical P supply for triggering the P-starvation response of root, including morphology, AM colonization rate, acid phosphatase activity in rhizosphere soil, and expression levels of genes encoding Pi transporter, phosphatase, ribonucleases, and expansin. This research provides useful information for developing soil-based and rhizosphere-based P management with the aims of improving both high yield and efficient P use.
Materials and methods
Field experiments
Two consecutive field experiments were carried out during the growing seasons of 2009–2010 (2010 experiment) and 2010–2011 (2011 experiment) at Quzhou experimental station (36.5° N 115.0° E, 40 m above sea level) of China Agricultural University. The climate in the region is warm and subhumid and consists of summer rainfall and dry cold winters. The average annual temperature is 13.2 °C. According to data from 1980 to 2011, annual precipitation ranges from 213 to 840mm (mean 494mm). The precipitation during the wheat-growing season was 149.3 and 62.5mm in the 2010 and 2011 experiments, respectively. The soil at the study site is a silt fluvo-aquic soil. The basic soil properties in 0–30cm layer are given in Supplementary Table S1 (available at JXB online). Triticum aestivum L. cv. Kenong 9204 was used in both 2010 and 2011, sown on 8 October 2009 and 7 October 2010 and harvested on 14 June 2010 and 11 June 2011, respectively.
Both experiments were treated with six P supplies, 0, 25, 50, 100, 200, and 400kg ha–1 of P as calcium superphosphate (referred as P0, P25, P50, P100, P200, and P400, respectively). All treatments received 225kg ha–1 of N as urea split into 75kg ha–1 before sowing and 150kg ha–1 at stem elongation (Feekes6.0). P fertilizer and 60kg ha–1 of K2O as potassium sulphate were added to the topsoil before sowing by broadcast application and then mixed by conventional tillage (0–30cm depth). Each P treatment was replicated four times in a randomized complete block design with plot size 43.2 m2 (5.4×8 m). Seeds were sown at a rate of 375 seeds m–2 with 20-cm row spaces in the 2010 experiment and 15-cm row spaces in the 2011 experiment. For both experiments, the sowing depth was 3–4cm and irrigation was with ~90mm underground water per irrigation before winter, during stem elongation, and near anthesis. Weeds were well controlled by manual removal. Pests and diseases were controlled by spraying insecticide (cypermethrin) and fungicide (carbendazim) before stem elongation and after flowering.
At flowering (Feekes10.5.2), shoot biomass yield, shoot P concentration, root dry weight (RDW), root length density (RLD), AM colonization rate, acid phosphatase activity in rhizosphere soil (RS-APase), and gene expression in shoots and roots were investigated. Shoot biomass yield was recorded by sampling the aboveground parts of two rows (0.5 m long per row) and drying to a constant weight at 60 °C.
After the aboveground parts were removed, the underground parts were used to determine RDW, RLD, and root AM colonization rate. To measure these root traits, soil volumes of 40×20cm to a total depth of 60cm with 10-cm increments in each plot of all six P treatments were dug out in the 2010 experiment, and a soil volume of 30×10cm to a total depth of 60cm with 10-cm increments in each plot of four P treatments (P0, P50, P100, and P400) were dug out in the 2011 experiment. Thus, there were six soil blocks of 40 20 10cm in each plot in 2010 and six soil blocks of 30 10 10cm in each plot in 2011. All visible roots in each soil block were picked out in the field by hand and placed in individual, marked plastic bags. These roots were washed free of soil after transfer to the laboratory and then frozen at –20 °C until RLD and AM colonization analysis.
To measure gene expression levels and RS-APase, 10 randomly selected plants with roots 30cm in depth were collected at flowering in each plot. The flag leaves of nine other plants were sampled together and stored in liquid N2 for gene expression analysis. Each root sample was randomly divided into two subsamples. One subsample was used for measurement of APase activity in the rhizosphere soil. The other subsample was quickly washed free of soil and both adventitious root and seminal roots were collected and stored in liquid N2 until gene expression analysis.
At maturity (Feekes11.4), the whole plot was harvested to measure biomass yield, grain yield, and total P in the straw and grain.
Soil Olsen P and CaCl2-P concentrations were also monitored at flowering and maturity. Five subsamples per plot were collected from the topsoil (0–30cm) between wheat rows. The subsamples were then thoroughly mixed and air dried before being ground to analyse soil Olsen P and CaCl2-P.
Determination of plant total P and soil Olsen P and CaCl2-P
To determine plant total P, dried samples were milled and subsequently digested with concentrated H2SO4 and H2O2 for determining total P using the molybdate-blue colorimetric method (Murphy and Riley, 1962). Soil Olsen P was determined using the molybdo-vanadophosphatase method based on extraction from air-dried soil with 0.5M NaHCO3 at pH 8.5 (180rpm, 25 °C) (Westerman, 1990). CaCl2-P was extracted using 0.01M CaCl2 at a soil/solution ratio of 1:5 (Schofield, 1955) and determined by the molybdate-blue colorimetric method. Absorbance was recorded on a 4802 UV/VIS double beam spectrophotometer (UNICO, Shanghai, China).
Scanning and image analysis of roots
Cleaned root samples were dispersed in water in a transparent array (30×20×3cm) and scanned (Epson Expression 1600, Seiko Epson, Nagano, Japan) at a resolution of 400 dpi. To evaluate root length, the images were analysed using WinRHIZO software (Regent Instrument, Quebec, Canada). After scanning, the root samples were oven dried and weighed. After calculation of the total root length and soil volume, the RLD was obtained.
Estimation of root AM colonization
To investigate root AM colonization, subsamples of root systems were randomly selected from the root samples collected at 0–60cm soil depth at flowering and treated and stained with nonvital trypan blue (Phillips and Hayman, 1970) as described by Feng et al. (2003) with some modifications. Stained roots were observed with a microscope and the intensity of root cortex colonization by AM fungi was determined as described by Trouvelot et al. (1986) using MYCOCALC software (www.dijon.inra.fr/mychintec/Mycocalc-prg/download.html).
Determination of acid phosphatases in rhizosphere soil
To measure RS-APase activity in the rhizosphere soil, roots were lifted out of the soil and shaken gently to remove loosely adhering soil, only leaving a proportion of tightly adhering soil, which was considered as rhizosphere soil. The roots with adhering rhizosphere soil were immersed into 50ml of 0.2mM CaCl2 and shaken carefully. RS-APase activities in the rhizosphere soil suspensions were determined by the method of Neumann (2006).
RNA extraction and quantitative real-time PCR
Total RNA in the plant samples was extracted using Trizol reagent (15596018, Invitrogen, USA), treated with RNase-free DNase (79254, Qiagen, Germany), and further purified with a RNeasy Plant Mini kit (74904, Qiagen) according to the manufacturer’s instructions. cDNA synthesis was performed using a PrimeScript RT Perfect Real Time reagent kit (DRR037A, Takara, Dalian) according to the manufacturer’s protocol. Quantitative real-time PCR was performed on a Mastercycler Realplex4 Real Time PCR System (Eppendorf, Germany) using SYBR Premix EX Taq (DRR041A, Takara) in 20-μl reaction volumes, which contained 10 μl SYBR Green PCR mix, 0.4μM each forward and reverse primers, 0.4μg diluted cDNA template, and the appropriate amounts of sterile double-distilled water. The primer sequences are listed in Supplementary Table S2. Levels of transcription were calculated with the 2-ΔΔCt method using the wheat housekeeping gene Actin as an internal control. All reactions were set up using four biological replicates.
Statistical analysis
All data were calculated using Excel 2003 (Microsoft, USA), and results are presented as mean ± standard error. Analysis of variance was performed using the one-way analysis of variance (ANOVA) model in the SAS statistical software (SAS Institute, Cary, NC, USA). Comparisons of means were performed using Duncan’s multiple range analysis test (α = 0.05).
Results
Plant growth and P uptake
P fertilizer significantly increased Olsen P in the bulk soils sampled at flowering and maturity in both experiments (Tables 1 and 2). CaCl2-P in bulk soil was only investigated at flowering. In both experiments, CaCl2-P was low when P-fertilizer rate was lower than P100 and then increased when P-fertilizer rate increased (Table 1). Biomass yield at flowering and maturity increased with P-fertilizer rate at first, but did not significantly increase further when P-fertilizer rate exceeded P100 (Tables 1 and 2). P fertilizer significantly increased shoot P at flowering and grain P at maturity in both experiments (Tables 1 and 2). The increasing effect of P fertilizer on straw P at maturity was observed in both experiments with P-fertilizer rate increased to P100 but not significantly at higher P supplies (Table 2). P uptake in terms of total P accumulated in the aboveground parts at flowering and maturity was calculated. At flowering and maturity, P uptake increased with P-fertilizer rate at first, and then did not significantly increase further when P-fertilizer rate was higher than P100 in both experiments (Tables 1 and 2). These results show that P100 is a critical P supply for most agronomic and P-uptake-related traits under these experimental conditions.
Table 1.
Soil Olsen-P, CaCl2-P, shoot biomass, P concentration, P uptake, and root traits under different P supplies at flowering in the field experiments. Data are means of four biological replicates. AM, arbusular-mycorrhizal; APase, acid phosphatase. Statistical differences (P < 0.05) between P supplies are indicated by different superscript letters.
| Trait | P supply (kg P ha–1) | |||||
|---|---|---|---|---|---|---|
| 0 | 25 | 50 | 100 | 200 | 400 | |
| 2010 experiment | ||||||
| Olsen-P (mg kg–1) | 13.3d | 13.9d | 17.9d | 25.8c | 45.6b | 83.8a |
| CaCl2-P (mg kg–1) | 0.07b | 0.08b | 0.04b | 0.12b | 0.45b | 2.41a |
| Shoot biomass yield (Mg ha–1) | 3.89c | 4.60b,c | 5.48b | 6.89a | 7.21a | 7.01a |
| Shoot P (mg g–1) | 2.28c | 2.26c | 2.31c | 2.42b,c | 2.76a,b | 2.80a |
| P uptake (kg ha–1) | 8.96b | 10.36b | 12.63b | 16.53a | 19.94a | 19.68a |
| Root dry weight (g m–3) | 105.2b | 106.6b | 121.7a,b | 152.5a | 133.9a,b | 121.2a,b |
| Root length density (cm cm–3) | 2.38b | 2.71a,b | 2.68a,b | 3.62a | 3.21a,b | 2.80a,b |
| AM colonization rate (%) | 36.51a | 33.22a | 14.94b | 8.40c | 6.59c | 2.43c |
| 2011 experiment | ||||||
| Olsen-P (mg kg–1) | 3.8d | 7.4c,d | 8.9c,d | 16.5c | 29.0b | 46.6a |
| CaCl2-P (mg kg–1) | 0.09b | 0.08b | 0.10b | 0.16a,b | 0.42a,b | 0.53a |
| Shoot biomass yield (Mg ha–1) | 5.22d | 7.20c,d | 7.88b,c,d | 10.91a | 10.29a,b | 9.64a,b,c |
| Shoot P (mg g–1) | 1.46d | 1.65c,d | 1.82b,c | 2.04a,b | 2.13a,b | 2.30a |
| P uptake (kg ha–1) | 5.49c | 11.93b | 14.25b | 24.90a | 24.10a | 22.13a |
| Root dry weight (g m–3) | 143.1a | – | 155.5a | 175.3a | – | 160.3a |
| Root length density (cm cm–3) | 2.34a | – | 2.59a | 3.09a | – | 2.98a |
| Rhizosphere APase (μg PNP g–1 soil h–1) | 343.1a,b | 325.0b | 338.9a,b | 427.60a | 225.7c | 163.7c |
| AM colonization rate (%) | 52.68a | 42.29b | 37.73b | 21.51c | 10.87d | 11.42d |
Table 2.
Soil Olsen-P, shoot biomass, grain yield, P concentration, and P uptake under different P supplies at maturity in the field experiments. Data are means of four biological replicates. Statistical differences (P < 0.05) between P supplies are indicated by different superscript letters.
| Trait | P supply (kg P ha–1) | |||||
|---|---|---|---|---|---|---|
| 0 | 25 | 50 | 100 | 200 | 400 | |
| 2010 experiment | ||||||
| Olsen-P (mg kg–1) | 6.7c | 11.5c | 12.0c | 19.5b,c | 44.1a,b | 55.8a |
| Grain yield (Mg ha–1) | 3.18d | 4.12c | 4.42b,c | 5.24a,b | 5.92a | 5.66a |
| Shoot biomass yield (Mg ha–1) | 7.36d | 8.24c,d | 9.81b,c | 10.90a,b | 12.54a | 12.61a |
| Straw P (mg g–1) | 0.35d | 0.40b,c,d | 0.37c,d | 0.48a,b,c | 0.49a,b | 0.55a |
| Grain P (mg g–1) | 3.29c | 3.40c | 3.52b,c | 3.56b,c | 3.68a,b | 3.87a |
| P uptake (kg ha–1) | 12.88d | 15.71c,d | 19.15b,c | 21.41a,b | 25.17a | 25.68a |
| 2011 experiment | ||||||
| Olsen-P (mg kg–1) | 4.7e | 6.5e | 9.6d | 17.2c | 32.7b | 53.6a |
| Grain yield (Mg ha–1) | 3.43c | 4.86b | 5.77a,b | 5.89a,b | 6.33a | 6.80a |
| Shoot biomass yield (Mg ha–1) | 6.80d | 9.35c | 10.77b,c | 11.68a,b | 12.23a,b | 13.41a |
| Straw P (mg g–1) | 0.22b | 0.26b | 0.30b | 0.39a | 0.42a | 0.43a |
| Grain P (mg g–1) | 2.68c | 2.97b,c | 3.14a,b | 3.38a | 3.45a | 3.37a |
| P uptake (kg ha–1) | 11.75d | 15.63c,d | 18.13b,c | 22.16a,b | 24.28a | 25.72a |
Estimation of optimal P supply for maximum grain yield
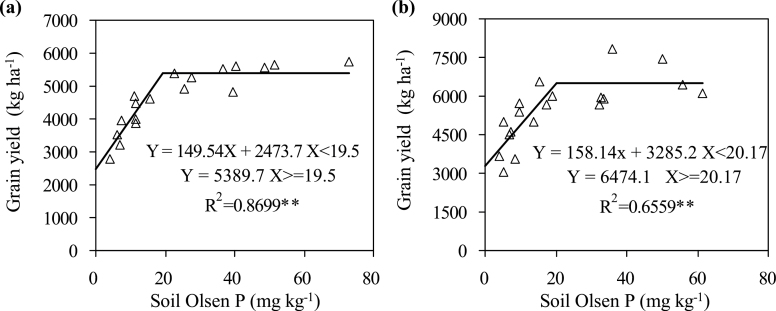
This study observed a statistically significant response of grain yield to P-fertilizer rate in both experiments (Table 2). To estimate the optimal P supply for maximum grain yield, grain yield was plotted against soil Olsen P measured at maturity. Regression analysis revealed that the response of grain yield to soil Olsen P significantly fitted to a linear-plateau model in both experiments (Fig. 1). The regression equations clearly showed that the grain yield reached its highest at Olsen P 19.5mg kg–1 in the 2010 experiment (Fig. 1A) and 20.17mg kg–1 in the 2011 experiment (Fig. 1B).
Fig. 1.
Grain yield as a function of increasing soil Olsen P in the 2010 (A) and 2011 (B) field experiments.** P < 0.01.
Estimation of critical P supply for P-starvation response based on P-starvation marker gene expression analysis
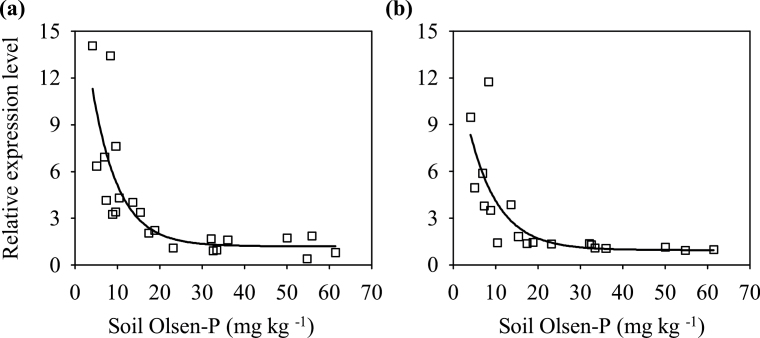
TaIPS1.1 belongs to the MT4/TPS1 family, members of which have been used as molecular indicators of plant Pi status (Shin et al., 2006; Huang et al., 2011). Here TaIPS1.1 was used as an indicator of Pi status of wheat plants. In both roots and shoots in the 2011 experiment, the change point for the response of TaIPS1.1 to P supply was approximately soil Olsen P 20mg kg–1 (Fig. 2).
Fig. 2.
Response of TaIPS1.1 relative expression level to soil Olsen P in shoots (A) and roots (B) in the 2011 experiment.
The response of both grain yield and TaIPS1.1 to P supply changed at approximately Olsen P 20mg kg–1 (Figs. 1 and 2), which corresponded to P100 in both experiments (Tables 1 and 2). In order to subsequently conveniently describe the responses of root traits to P supply, P0, P25, and P50 were classified as deficiency, P100 as optimum, and P200 and P400 as excess.
Responses of root morphology and physiology to P supply
RDW, RLD, and root AM colonization rate was investigated in 0–60cm depth soil and RS-APase activity was investigated in the 30-cm soil layer at flowering. In both experiments, RDW and RLD increased with P-fertilizer rate at first, peaked at P100, and then declined (Table 1). However, the statistically significant effects of P fertilizer on RDW and RLD were observed only in the 2010 experiment. P fertilizer decreased root AM colonization rate in both experiments. In the 2010 experiment, the root AM colonization rate was maintained below 9% at the sufficient P-fertilizer rates (optimum and excess) and significantly increased when P-fertilizer rate decreased from sufficiency to deficiency (Table 1). In the 2011 experiment, the AM colonization rate was maintained at about 10% at the excessive P-fertilizer rate and increased steadily when P-fertilizer rate decreased from excess to optimum and deficiency (Table 1). RS-APase activity was measured in the 2011 experiment and the two excessive P-fertilizer rates had lower RS-APase activity than the deficient and optimal P-fertilizer rates (Table 1).
Responses of P-starvation response genes to P supply
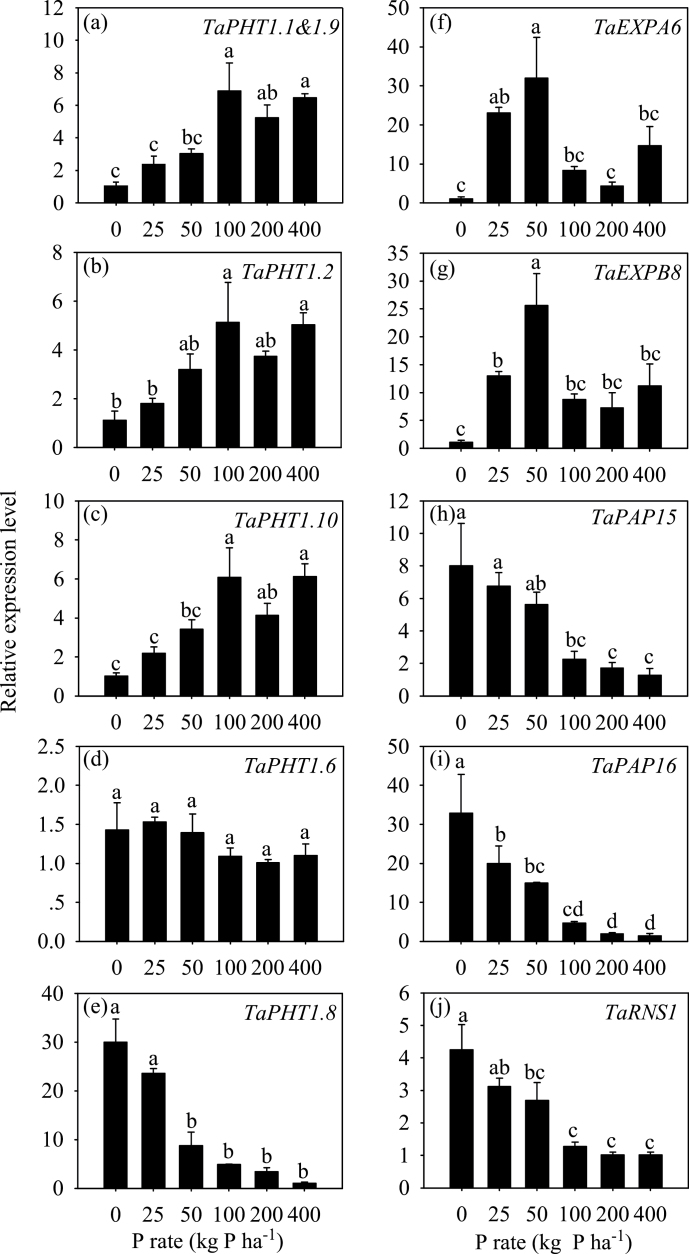
In the 2011 experiment, the expression levels of 11 PSI genes were investigated in the roots at flowering, including six PHT1 transporters, two expansin genes (TaEXPA6 and TaEXPB8), two purple phosphatase genes (TaPAP15 and TaPAP16), and one ribonuclease gene (TaRNS1) (Fig. 3). The expression levels of TaPHT1.1 and -1.9, TaPHT1.2, and TaPHT1.10 showed inhibitory responses to P deficiency, as they were maintained relatively high at the sufficient P-fertilizer rates and were downregulated steadily when P-fertilizer rate was reduced to deficiency (Fig. 3A–C). The expression of TaPHT1.6 did not show a significant response to P supply (Fig. 3D). The expression levels of TaPHT1.8, TaPAP15, TaPAP16, and TaRNS1 exhibited inducible responses to P deficiency, as they were kept at a low level at the sufficient P-fertilizer rates and were upregulated steadily when P-fertilizer rate was reduced to deficiency (Fig. 3E, H–J). The expression levels of TaEXPA6 and TaEXPB8 showed combined inducible and inhibitory responses to P deficiency, as they were upregulated when P-fertilizer rate decreased from sufficiency to P50 and were downregulated when P-fertilizer rate decreased from to P25 and P0 (Fig. 3F, G).
Fig. 3.
Relative expression levels at flowering of P-starvation response genes in roots of wheat grown under different P supplies in 2011 field experiment. (A–E) PHT1 Pi transporters PHT1.1 and 1.9, PHT1.2, PHT1.6, PHT1.8, and PHT1.10, respectively; (F) α-expansin EXPA6; (G) β-expansin EXPB8; (H and I) purple acid phosphatase genes PAP15 and PAP16, respectively; (J) ribonuclease gene RNS1. Data are mean ± standard error of four replicates. Different letters indicate significant differences between different P supplies (P = 0.05).
Discussion
P fertilizer promotes plant growth and P uptake
P fertilizer significantly increased biomass yield, grain yield, total P in the investigated plant tissues, and P uptake in both field experiments, but the values of most of these traits did not significantly increase further when P-fertilizer rate exceeded P100 (Tables 1 and 2, Fig. 1). These results suggested that these two experiments were suitable for estimating the responses of the above- and underground parts to P supply covering deficiency, optimum, and excess. Soil Olsen P increased with P-fertilizer rate, while CaCl2-P increased only when P-fertilizer rate exceeded P100 (Table 1), suggesting that P-fertilizer rate higher than 100kg ha–1 may environmental risks.
The optimal P supply for maximum yield coincides with the change point for the response of P-starvation marker gene to P supply
This study estimated the optimal P supply for maximum yield based on the yield responses to soil Olsen P measured at maturity. Soil Olsen P about 20mg kg–1 was optimal for achieving the highest grain yield in both experiments (Fig. 1). This is in the range 4.9–20.0mg kg–1 that has been reported for wheat previously (Jackson et al., 1997; Bollons and Barraclough 1999; Tang et al., 2009) and is also in the range of target soil Olsen P (14–30mg kg–1) for winter wheat in the north China Plain based on the P management strategy ‘building-up and maintenance method’ (Li et al., 2011a).
To determine the critical P supply for P-starvation response, this study then analysed the expression levels of TaIPS1.1 in shoots and roots in the 2011 experiment. The response pattern of TaIPS1.1 to P supply was similar in shoots and roots and had the change point Olsen P 20mg kg–1 (Fig. 2), which corresponds to P100 (Tables 1 and 2). As TaIPS1.1 belongs to the MT4/TPS1 family, members of which are used as P-starvation marker genes to reflect plant Pi status (Shin et al., 2006; Huang et al., 2011), Olsen P 20mg kg–1 also indicated the critical P supply for wheat plants to absorb sufficient P under these experimental conditions. This was supported by the fact that shoot P at flowering increased with increasing P-fertilizer rate at first and then did not increase further when P-fertilizer rate increased from P100 to higher rates (Table 1).
As already discussed, the optimal P supply for maximum yield coincided with the critical P concentration for P-starvation marker gene response in roots and shoots. Since P-deficiency-induced changes in root morphology, physiology, and biochemistry are coordinated by a small number of regulatory systems controlled by both plant P status and soil Pi availability (George et al., 2011), this study then checked whether P100, which produced soil Olsen P approximately 20mg kg–1, was critical for the P-starvation responses. As shown in detail in the following discussion, the responses of most root traits to P supply changed at or near P100 (Table 1, Fig. 3). These results are supported by previous studies. For instance, a study on pasture species including grasses and dicots also observed that root morphological adaptations were triggered at or near the critical P supply for shoot mass, although it was carried out under controlled conditions (Hill et al., 2006).
Optimal P supply for crop production has been estimated worldwide in a number of crops. Thus, the coincidence between the optimal P supply for yield performance and the change points for the responses of root traits to P supply is that the numerous information about optimal P supply is significant because it could be used to predict the root processes related with P acquisition according to soil P supply, although the coincidence is needed to be investigated in more soil types and crop genotypes. Considering the difficulty in observing root processes in soil, the prediction of root processes is very useful for optimizing soil-based and rhizosphere-based P managements targeting both high yield and efficient P use.
Root processes respond differentially to P supply
In response to low Pi availability, plant roots adaptively change their morphology, physiology, and biochemistry, including enhanced adventitious rooting and lateral root branching, greater root hair and cluster root formation, increased mobilization of sparse soluble soil/fertilizer P by root exudates such as phosphatase and ribonuclease enzymes, upregulated activity of Pi transporters, and increased symbiotic association with AM fungi (Vance et al., 2003; Lambers et al., 2006; Shen et al., 2011). However, the investigated root traits responded to P supply in contrasting patterns under the current experimental conditions. These root traits included RDW, RLD, root AM colonization rate, RS-APase activity, and expression levels of 11 PSI genes encoding PHT1 Pi transporter, purple acid phosphatase, ribonuclease, and expansin, and therefore reflected the morphological, physiological, and biochemical properties of roots.
The traits that were induced by P deficiency included root AM colonization rate (Table 1), RS-APase activity (Table 1), and expression levels of TaPHT1.8, TaPAP15, TaPAP16, and TaRNS1 (Fig. 3E, H–J). The critical P supply for P-deficiency response was P100 for root AM colonization rate in the 2010 experiment, and was P100 for TaPAP15, TaPAP16, and TaRNS1 expression, P50 for TaPHT1.8, and P200 for root AM colonization rate and RS-APase activity in the 2011 experiment. TaPHT1.8 (known as TaPHT;myc) has been shown to be specifically upregulated in roots colonized by AM fungi, indicating its function in Pi transport via a mycorrhizal-dependent pathway (Glassop et al., 2005). Therefore, upregulation of TaPHT1.8 by P deficiency would be the result of increased root AM colonization rate under P deficiency. This study observed that RS-APase activity was higher under P-deficient than under P-excessive conditions (Table 1), implying that genes encoding phosphatase were upregulated in the roots under P deficiency. This was supported by the upregulation of two purple acid phosphatase genes (TaPAP15, TaPAP16) in the roots by P deficiency (Fig. 3H, I). However, roots had relatively high RS-APase activity but relatively low levels of TaPAP15 and TaPAP16 mRNA at P100 (Table 1, Fig. 3H, I); this conflict might have been caused by the method of measuring RS-APase, which could not avoid contamination with organic compounds from damaged roots and microorganisms.
The traits that exhibited inhibitory response patterns to P deficiency included the expression of TaPHT1.1 and -1.9, TaPHT1.2, and TaPHT1.10 (Fig. 3A–C). However, these four genes have been shown to be induced by P deficiency in a previous study (Davies et al., 2002) and a preliminary study (data not shown), and their orthologues in a close relative of wheat, barley (Hordeum vulgare L.), are also induced by P deficiency (Huang et al., 2011). The inhibition of these four wheat genes by P deficiency could be, at least partially, explained by the upregulated AM colonization under P deficiency (Table 1). Phylogenetic analysis of PHT1 proteins showed that these four genes are closely related to barley HvPHT1.1 and HvPHT1.9 (Supplementary Fig. S1). In barley, AM colonization has been found to inhibit the response of HvPHT1.1 and HvPHT1.2 to P deficiency in roots (Glassop et al., 2005). TaPHT1.6 showed a poor response to P supply and was not inhibited by P deficiency (Fig. 3D). TaPHT1.6 has been found to respond poorly to P deficiency in the roots of some wheat genotypes (Davies et al., 2002).
The two expansin genes TaEXPA6 and TaEXPB8 demonstrated combined inducible and inhibitory responses to P deficiency, as their transcripts increased when P-fertilizer rate decreased from sufficiency to P50 and then declined when P-fertilizer rate decreased from P50 to lower concentrations (Fig. 3F, G). Expansins are proteins that promote cell-wall loosening and extension and they function in diverse aspects of plant growth and development, including root development (Choi et al., 2006). For example, a root-specific and P-deficiency-inducible β-expansin, GmEXPB2, was found to promote root development and enhance plant growth and P uptake under both low and high P (Guo et al., 2011). The α-expansin gene TaEXPA6 and β-expansin gene TaEXPB8 were found to predominantly expressed in roots in a previous study (Lin et al., 2005) and were induced by P deficiency in a preliminary study (data not shown), implying their possible functions in P-deficiency-induced root morphological changes. The response patterns of TaEXPA6 and TaEXPB8 to P supply were similar to, and could be at least partially explained by, the response patterns of RDW and RLD to P supply in that RDW and RLD peaked at P100 in the 2010 experiment (Table 1), while TaEXPA6 and TaEXPB8 reached their highest mRNA levels at P50 (Fig. 3F, G). In the 2011 experiment, RDW and RLD did not exhibit a significant response to P supply, but RDW and RLD at P0 were much lower than at P100 (Table 1), reflecting the inhibition of P0 on the expression levels of TaEXPA6 and TaEXPB8 (Fig. 3F, G). The inhibition of P deficiency on root development has been shown for both hydroponically grown and field-grown wheat plants (Sun and Zhang et al., 2000; Hu et al., 2010).
In summary, this study characterized the root processes at six P supplies by investigating RDW, RLD, AM colonization rate, RS-APase activity, and expression levels of genes regulating Pi transporter, phosphatase, and root development. Although these processes are thought to increase the ability of the root systems to utilize Pi from soils in the published literature, several of these root traits were inhibited by P deficiency under the field conditions in this study. Therefore, more detailed research is needed to better understand the mechanisms of plant adaptation to soil P dynamics under field conditions. This study also observed that the optimal P supply for maximum grain yield coincided with the critical P supply for the response of P-starvation response marker genes to P deficiency and was at or near the change points for response of root processes to P supply. These results improve understanding of the molecular significance of optimal P supply for maximum yield and also provide useful information for optimizing P-management strategies based on the soil and the plant rhizosphere.
Supplementary material
Supplementary data are available at JXB online.
Supplementary Table S1. Basic soil properties in 0–30cm soil layer before the field experiments.
Supplementary Table S2. Quantitative real-time PCR primers.
Supplementary Fig. S1. Phylogenetic analysis of PHT1 proteins from wheat, barley, rice, and maize.
Acknowledgements
This research was supported by the National Natural Science Foundation of China (30890133 and 30971872) and the Ministry of Science and Technology of China (2011CB100304 and 2009CB118302).
References
- Ai P, Sun S, Zhao J, et al. 2009. Two rice phosphate transporters, OsPht1;2 and OsPht1;6, have different functions and kinetic properties in uptake and translocation. The Plant Journal 57, 798–809 [DOI] [PubMed] [Google Scholar]
- Bollons HM, Barraclough PB. 1999. Assessing the phosphorus status of winter wheat crops: inorganic orthophosphate in whole shoots. Journal of Agricultural Science 133, 285–295 [Google Scholar]
- Bucher M. 2007. Functional biology of plant phosphate uptake at root and mycorrhiza interfaces. New Phytologist 173, 11–26 [DOI] [PubMed] [Google Scholar]
- Burleigh SH, Harrison MJ. 1998. Characterization of the Mt4 gene from Medicago truncatula. Gene 216, 47–53 [DOI] [PubMed] [Google Scholar]
- Choi DS, Cho HT, Lee Y. 2006. Expansins: expanding importance in plant growth and development. Physiologia Plantarum 126, 511–518 [Google Scholar]
- Cordell D, Drangert JO, White S. 2009. The story of phosphorus: global food security and food for thought. Global Environmental Change 19, 292–305 [Google Scholar]
- Davies TGE, Ying J, Xu Q, Li ZS, Li J, Gordon-Weeks R. 2002. Expression analysis of putative high-affinity phosphate transporters in Chinese winter wheats. Plant, Cell and Environment 25, 1325–1339 [Google Scholar]
- Food and Agriculture Organization 2006. Fertilizer use by crop. Fertilizer and plant nutrition bulletin 17. Rome: FAO; [Google Scholar]
- Feng G, Song YC, Li LX, Christie P. 2003. Contribution of arbuscular mycorrhizal fungi to utilization of organic sources of phosphorus by red clover in a calcareous soil. Applied Soil Ecology 22, 139–148 [Google Scholar]
- George TS, Fransson AM, Hammond JP, White PJ. 2011. Phosphorus nutrition: rhizosphere processes, plant response and adaptations. In: Bünemann E, Oberson A, Frossard E, eds, Phosphorus in action: soil biology, vol. 100, Part 2, Dordrecht: Springer, pp 245–271 [Google Scholar]
- George TS, Richardson AE. 2008. Potential and limitations to improving crops for enhanced phosphorus utilization. In: Hammond JP, White PJ, eds, The ecophysiology of plant –phosphorus interactions. Dordrecht: Springer, pp 247–270 [Google Scholar]
- Glassop D, Smith SE, Smith FW. 2005. Cereal phosphate transporters associated with the mycorrhizal pathway of phosphate uptake into roots. Planta 222, 688–698 [DOI] [PubMed] [Google Scholar]
- Guo WB, Zhao J, Qin L, Yan XL, Liao H. 2011. A soybean β-expansin gene GmEXPB2 intrinsically involved in root system architecture responses to abiotic stresses. The Plant Journal 66, 541–552 [DOI] [PubMed] [Google Scholar]
- Hammond JP, White PJ. 2008. Sucrose transport in the phloem: integrating root responses to phosphorus starvation. Journal of Experimental Botany 59, 93–109 [DOI] [PubMed] [Google Scholar]
- Hermans C, Hammond JP, White PJ, Verbruggen N. 2006. How do plants respond to nutrient shortage by biomass allocation? Trends in Plant Science 11, 610–617 [DOI] [PubMed] [Google Scholar]
- Hill JO, Simpson RJ, Moore1 AD, Chapman DF. 2006. Morphology and response of roots of pasture species to phosphorus and nitrogen nutrition. Plant and Soil 286, 7–19 [Google Scholar]
- Hinsinger P. 2001. Bioavailability of soil inorganic P in the rhizosphere as affected by root induced chemical changes: a review. Plant and soil 237, 173–195 [Google Scholar]
- Hinsinger P, Bengough AG, Vetterlein D, Young IM. 2009. Rhizosphere: biophysics, biogeochemistry, and ecological relevance. Plant and soil 321, 117–152 [Google Scholar]
- Hu JL, Lin XG, Wang JH, Cui XC, Dai J, Chu HY, Zhang JB. 2010. Arbuscular mycorrhizal fungus enhances P acquisition of wheat (Triticum aestivum L.) in a sandy loam soil with long-term inorganic fertilization regime. Applied Microbiology and Biotechnology 88, 781–787 [DOI] [PubMed] [Google Scholar]
- Huang CY, Shirley N, Genc Y, Shi BJ, Langridge P. 2011. Phosphate utilization efficiency correlates with expression of low-affinity phosphate transporters and noncoding RNA, IPS1, in barley. Plant Physiology 156, 1217–1229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson GD, Kushnak GD, Carlson GR, Wichman DM. 1997. Correlation of the Olsen phosphorus soil test: spring wheat response. Communications in Soil Science and Plant analysis 28, 813–822 [Google Scholar]
- Jain A, Vasconcelos MJ, Raghothama KG, Sahi SV. 2007. Molecular mechanisms of plant adaptation to phosphate deficiency. Plant Breeding Reviews 29, 359–419 [Google Scholar]
- Jing J, Rui Y, Zhang F, Rengel Z, Shen J. 2010. Localized application of phosphorus and ammonium improves growth of maize seedlings by stimulating root proliferation and rhizosphere acidification. Field Crops Research 119, 355–364 [Google Scholar]
- Jones DL, Dennis PG, Owen AG, van Hees PAW. 2003. Organic acid behavior in soils-misconceptions and knowledge gaps. Plant and Soil 248, 31–41 [Google Scholar]
- Kirkby EA, Johnston AE. 2008. Soil and fertilizer phosphorus in relation to crop nutrition. In: Hammond JP, White PJ, eds, The ecophysiology of plant–phosphorus interactions. Dordrecht: Springer, pp 177–223 [Google Scholar]
- Lambers HY, Shane MW, Cramer MD, Pearse SJ, Veneklass EJ. 2006. Root structure and functioning for efficient acquisition of phosphorus: matching morphological and physiological traits. Annals of Botany 98, 693–713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Huang G, Meng Q, et al. 2011a. Integrated soil and plant phosphorus management for crop and environment in China. Plant and Soil 349, 157–167 [Google Scholar]
- Li Z, Gao Q, Liu Y, He C, Zhang X, Zhang J. 2011b. Overexpression of transcription factor ZmPTF1 improves low phosphate tolerance of maize by regulating carbon metabolism and root growth. Planta 233, 1129–1143 [DOI] [PubMed] [Google Scholar]
- Liang CY, Tian J, Lam HM, Lim BL, Yan XL, Liao H. 2010. Biochemical and molecular characterization of PvPAP3, a novel purple acid phosphatase isolated from common bean enhancing extracellular ATP utilization. Plant Physiology 152, 854–865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Z, Ni ZF, Zhang Y, Yao YY, Wu HY, Sun QX. 2005. Isolation and characterization of 18 genes encoding α- and β-expansins in wheat (Triticum aestivum L.). Molecular Genetics and Genomics 274, 548–556 [DOI] [PubMed] [Google Scholar]
- Liu C, Muchhal US, Mukatira U, Kononowicz AK, Raghothama KG. 1998. Tomato phosphate transporter genes are differentially regulated in plant tissues by phosphorus. Plant Physiology 116, 91–99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Bucio J, Vega OM, Guevara-Garia A, Herrera-Estrella L. 2000. Enhanced phosphorus uptake in transgenic tobacco plants that overproduce citrate. Nature Biotechnology 18, 450–453 [DOI] [PubMed] [Google Scholar]
- Lynch JP, Brown KM. 2008. Root strategies for phosphorus acquisition. In: Hammond JP, White PJ, eds, The ecophysiology of plant–phosphorus interactions. Dordrecht: Springer, pp 83–116 [Google Scholar]
- Marschner H. 1995. Mineral nutrition of higher plants, 2nd ed London: Academic Press; [Google Scholar]
- Murphy JR, Riley JP. 1962. A modified single solution method for the determination of phosphate in natural waters. Analytica Chimica Acta 27, 31–36 [Google Scholar]
- Neumann G. 2006. Quantitative determination of acid phosphatase activity in the rhizosphere and on the root surface by spectrophotometric assay. In: Luster J, Finlay R, eds. Handbook of methods used in rhizosphere research: online edition, available at http://www.rhizo.at/download.asp?id=1142 [Google Scholar]
- Péret B, Clémet M, Nussaume L, Desnos T. 2011. Root developmental adaptation to phosphate starvation: better safe than sorry. Trends in Plant Science 16, 442–450 [DOI] [PubMed] [Google Scholar]
- Phillips JM, Hayman DS. 1970. Improved procedures for clearing and staining parasitic and vesicular-arbuscular mycorrhizal fungi for rapid assessment of infection. Transactions of British Mycological Society 55, 158–161 [Google Scholar]
- Raghothama KG. 1999. Phosphate acquisition. Annual Review of Plant Physiology and Plant Molecular Biology 50, 665–693 [DOI] [PubMed] [Google Scholar]
- Raghothama KG. 2000. Phosphate transport and signalling. Current Opinion in Plant Biology 3, 182–187 [PubMed] [Google Scholar]
- Richardson AE, Hadobas PA, Hayes JE. 2001. Extracellular secretion of Aspergillus phytase from Arabidopsis roots enables plants to obtain phosphorus from phytate. The Plant Journal 25, 1–10 [DOI] [PubMed] [Google Scholar]
- Robinson D, 1994. The responses of plants to non-uniform supplies of nutrients. New Phytologist 127, 635–674 [DOI] [PubMed] [Google Scholar]
- Schofield RK. 1955. Can a precise meaning be given to ‘available’ soil phosphorus. Soils and Fertilisers 18, 373–375 [Google Scholar]
- Seo HM, Jung Y, Song S, et al. 2008. Increased expression of OsPT1, a high-affinity phosphate transporter, enhances phosphate acquisition in rice. Biotechnology Letters 30, 1833–1838 [DOI] [PubMed] [Google Scholar]
- Shen J, Yuan L, Zhang J, Li H, Bai Z, Chen X, Zhang W, Zhang F. 2011. Phosphorus dynamics: from soil to plant. Plant Physiology 156, 997–1005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin H, Shin HS, Chen RJ, Harrison MJ. 2006. Loss of At4 function impacts phosphate distribution between the roots and the shoots during phosphate starvation. The Plant Journal 45, 712–726 [DOI] [PubMed] [Google Scholar]
- Smith SE, Smith FA. 2011. Roles of arbusular mycorrhizas in plant nutrition and growth: new paradigma from cellular to ecosystem scales. Annual Review of Plant Biology 62, 227–250 [DOI] [PubMed] [Google Scholar]
- Sun HG, Zhang FS. 2000. Growth response of wheat roots to phosphorus deficiency. Journal of Integrative Plant Biology 42, 913–919 [Google Scholar]
- Tang X, Ma Y, Hao X, Li X, Li J, Huang S, Yang X. 2009. Determining critical values of soil Olsen-P for maize and winter wheat from long-term experiments in China. Plant and Soil 323, 143–151 [Google Scholar]
- Tian J, Wang X, Tong Y, Chen X, Liao H. 2012. Bioengineering and management for efficient phosphorous utilization in crops and pastures. Current Opinion in Biotechnology 23, 866–871 [DOI] [PubMed] [Google Scholar]
- Trouvelot A, Kough JL, Gianinazzi-Pearson V. 1986. Mesure du taux de mycorhization VA d’un système radiculaire. Recherche des méthodes d’estimation ayant une signification fonctionnelle. In: Gianinazzi-Pearson V, Gianinazzi S, eds, [Physiological and genetic aspects of mycorrhizae]. Paris: INRA Press, pp 217–221 [Google Scholar]
- Vance CP, Uhde-stone C, Allan DL. 2003. Phosphorus acquisition and use: critical adaptations by plants for securing a non renewable resource. New Phytologist 157, 423–427 [DOI] [PubMed] [Google Scholar]
- Wang X, Wang Y, Tian J, Lim B, Yan X, Liao H. 2009. Overexpressing AtPAP15 enhances phosphorus efficiency in soybean. Plant Physiology 151, 233–240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westerman RL. 1990. Soil testing and plant analysis, 3rded. Madison, WI: American Society of Agronomy and Soil Science Society of America; [Google Scholar]
- Zhang FS, Shen JP, Zhang JL, Zuo YM, Li L, Chen XP. 2010. Rhizosphere processes and management for improving nutrient use efficiency and crop productivity: implications for China. Advances in Agronomy 107, 1–32 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.