Abstract
Background
The photosensitizer pro-drug 5-aminolevulinic acid (5-ALA) has been administered systemically for photodynamic therapy. Although several toxicities have been reported, nephrotoxicity has never been observed.
Materials and Methods
Patients with head and neck mucosal dysplasia have been treated on a phase 1 study of escalating light doses in combination with 60 mg/kg of oral 5-ALA. Serum creatinine was measured with the modified Jaffe method or an enzymatic method in the first 24 hours after 5-ALA. Interference by 5-ALA, as well as by its photosensitizing product protoporphyrin IX, was assessed.
Results
Among 11 subjects enrolled to date, 9 of 11 had blood chemistries collected within the first 5 hours with 7 demonstrating significant grade 3 creatinine elevations (p=0.030). There was no additional evidence of compromised renal function or increased PDT-induced mucositis. Creatinine levels measured by the Jaffe assay increased linearly as a function of the ex-vivo addition of ALA (p<.0001). The exogenous addition of PpIX did not alter creatinine levels. ALA did not interfere with creatinine levels as measured by an enzymatic assay. A total of 4 of the 11 subjects had creatinine levels prospectively measured by both the Jaffe and the enzymatic assays. Only the Jaffe method demonstrated significant elevations as a function of time after ALA administration.
Conclusions
The transient increase in creatinine after systematic ALA can be attributed, in part, if not entirely, to interference of ALA in the Jaffe reaction. Alternative assays should be employed in situations calling for monitoring of kidney function after systemic ALA.
Keywords: Levulan, photodynamic therapy, side-effect, creatinine, Jaffe method
I. Introduction
Photodynamic therapy (PDT) with the photosensitizer pro-drug 5-aminolevulinic acid (5-ALA), the topical and oral formulation in Levulan®, has been described clinically for several indications including actinic keratosis1 and Barrett’s esophagus2. The exogenous administration of 5-ALA results in its endogenous conversion to the photosensitizing product, protoporphyrin IX (PpIX). It is an attractive drug of choice for use with PDT because of the selective accumulation of PpIX within neoplastic cells3, the short period of systemic cutaneous photosensitivity4, and its minimal and transient toxicities. Furthermore, inherent fluorescence enables noninvasive measurements of PpIX levels in tissue, which in turn provides real-time monitoring of drug activation and destruction as photoproducts and photobleaching, respectively5.
For these reasons, 5-ALA was selected for study in the management of dysplasia arising from the head and neck mucosa. During the course of study, we observed an unexpected rise in the serum creatinine shortly after administration of 5-ALA that has never been described in humans6. This report summarizes our observations and identifies a previously unknown interference between ALA and the widely used Jaffe method for measuring serum creatinine.
II. Material and Methods
Subjects were prospectively enrolled on a Phase I clinical trial of escalating light fluences in combination with a fixed oral dose of Levulan® (DUSA Pharmaceuticals, Wilmington, MA), the hydrochloride salt formulation of 5-ALA. The study was approved by the Institutional Review Board of the University of Pennsylvania and the University of Pennsylvania Clinical Trials Scientific Review and Monitoring Committee. Baseline CBC, electrolytes, BUN, creatinine, liver function tests, and PT/PTT were obtained before the administration of 5-ALA, as previous reports of adverse reactions associated with 5-ALA included elevated liver enzymes, nausea, vomiting, and skin photosensitivity. Repeat chemistry including renal and liver function tests were repeated at 24 hours after 5-ALA administration. The original study protocol was subsequently amended to systematically repeat serum chemistry including renal function tests at 1–2 hours and 4–5 hours after 5-ALA administration based on the results described in the Results Section. Laboratory tests were performed according to standard clinical pathology practices. In particular, serum creatinine concentration was measured by a modified rate Jaffe procedure7, as well as in Subjects 8–11 by an enzymatic method.
Photodynamic Therapy
On the day of the treatment, 60 mg/kg Levulan® dissolved in 50 ml water was orally administered 4–6 hours prior to light delivery. Vital signs were obtained immediately before and after drug administration, and at 15 minute intervals for the first two hours and then monitored every half-hour until the procedure. Vital signs remained within normal range throughout. Study subjects were brought to the operating room and placed under general anesthesia while maintaining light precautions to avoid inadvertent photoactivation of the upper airway mucosa. Intraoperative demarcation of the lesion borders were performed with either the use of pigmented operative towels or painting the mucosa with methylene blue. Light was delivered through a microlens (Medlight SA, Ecublens, Switzerland) or cylindrical diffusing fiber (Medlight SA) coupled to a Cerelas Series GaA1As 4 W diode laser (Biolitec, Inc., East Longmeadow, MA) that emits a peak wavelength of 632 nm and 90% of the power within 629–635 nm. The prescription was 50 or 100 J/cm2 delivered at 100 mW/cm2 as measured at the tissue surface by isotropic detectors. In some patients light was interrupted for 90 −180 sec after 20% of the dose had been delivered (fractionated delivery).
Light precautions were maintained during hospitalization, as significant skin and retina photosensitivity can persist up to 48 hours. Safety measures consisted of plastic light-absorbing filters placed on operating room lights and headlamps, draping of exposed skin during and after surgery, and minimization of exposure to outdoor, fluorescent, and incandescent light.
Creatinine Measurements after Ex Vivo Addition of ALA and/or PpIX
Serum creatinine levels were measured by both the Jaffe method and an enzymatic method. De-identified serum from patients with previously measured creatinine levels by the Jaffe method were pooled together into two samples: low (< 0.64) and high (> 4) creatinine. Serum was spiked with serial dilutions of either 5-aminolevulinic acid hydrochloride (Sigma, St. Louis, MO) dissolved in normal saline (Abbott Laboratories, Chicago, IL), or protoporphyrin IX disodium salt (Sigma, St. Louis, MO) dissolved in 100% DMSO (Sigma, St. Louis, MO). Final concentrations of DMSO and normal saline in the ex vivo samples were no greater than 0.5% and 0.2%, respectively.
Each serum sample was analyzed by both the Jaffe and enzymatic method. For the Jaffe method, samples were measured using the Beckman Coulter Unicel DxC 800 Synchron Analyzer (Beckman Instruments Inc., Fullerton, CA, USA). This system uses a standardized reagent (8.1 mmol/L picric acid buffered to an optimal pH of > 13.3) which measures serum creatinine concentration by a modified rate Jaffe method. In this reaction, creatinine in the sample reacts with picric acid in an alkaline solution to form an orange-red complex whose absorbance can be measured at 520 nm. The change in absorbance at this wavelength is directly proportional to the creatinine concentration in the sample7. For the enzymatic method, serum creatinine, was measured using the Nova Statprofile Critical Care Xpress analyzer (Nova Biomedical, Waltham, MA, USA)8. This platform uses 3 enzymes that catalyze the conversion of creatinine to ultimately form formaldehyde, glycine, and hydrogen peroxide. A potential of 0.70 volts is applied at the surface of a platinum anode which oxidizes electroactive hydrogen peroxide. The current generated by the flow of electrons at the surface of the electrode is proportional to the concentration of creatinine in the sample8.
Statistics
The effect of ex vivo addition of ALA and/or PpIX on results of the Jaffe or enzymatic assay was analyzed using linear regression to test the null hypothesis that ALA and/or PpIX were unassociated with the measured concentration of creatinine, i.e. the slope of the regression line was tested for a difference from zero. To examine the effect of ALA, separate regressions were carried out for the low creatinine concentration (Lo Cr) and high creatinine (Hi Cr) samples without PpIX. To examine the effect of PpIX, separate regressions were carried out for the Lo Cr and Hi Cr samples without ALA.
In patient samples, Friedman’s non-parametric test was used to test the global null hypothesis that creatinine levels were unchanged over time. Exact Wilson signed rank tests were then used to analyze changes in creatinine levels, as a function of specific times, For each of the four patients (subjects 8–11) with creatinine measured enzymatically at 3–4 time points post 5-ALA, we calculated means using all of the timepoints, and for three patients with variation in measured creatinine we constructed one-sided upper 95% confidence intervals (CI) to determine whether there was any evidence of elevated creatinine level. For the fourth patient, who demonstrated no variation in creatinine measured enzymatically, we used the median of the three observed standard deviations to construct the CI. R 2.109. was used for all analyses with a type I error rate of 0.05 for hypothesis testing. Precision of estimates are shown in parentheses as 95% confidence intervals.
III. Results
All subjects were treated for biopsy proven dysplasia ranging from moderate to severe dysplasia. In the first 3 enrolled subjects, serum creatinine levels 24 hours post 5-ALA ingestion were within normal range. Subject 3 received additional serum chemistry as this patient had a larynx primary site and remained intubated for potential airway precautions for the first 2 days after light administration. Because of a mildly elevated baseline serum potassium (5.2 mmol/L), subject 4 required a repeat chemistry. As a result, a 4.2-fold escalation in serum creatinine was incidently identified two hours following 5-ALA administration. Due to the unanticipated nature of this finding and, at the time, the lack of explanation for its cause, the treatment light was not delivered. No clinical complications or other aberrant laboratory values were noted in this subject to suggest direct renal injury. However, systematic renal investigations such as renal ultrasound or 24-hour creatinine clearance were not obtained as the repeat serum creatinine 24 hours after 5-ALA administration had normalized.
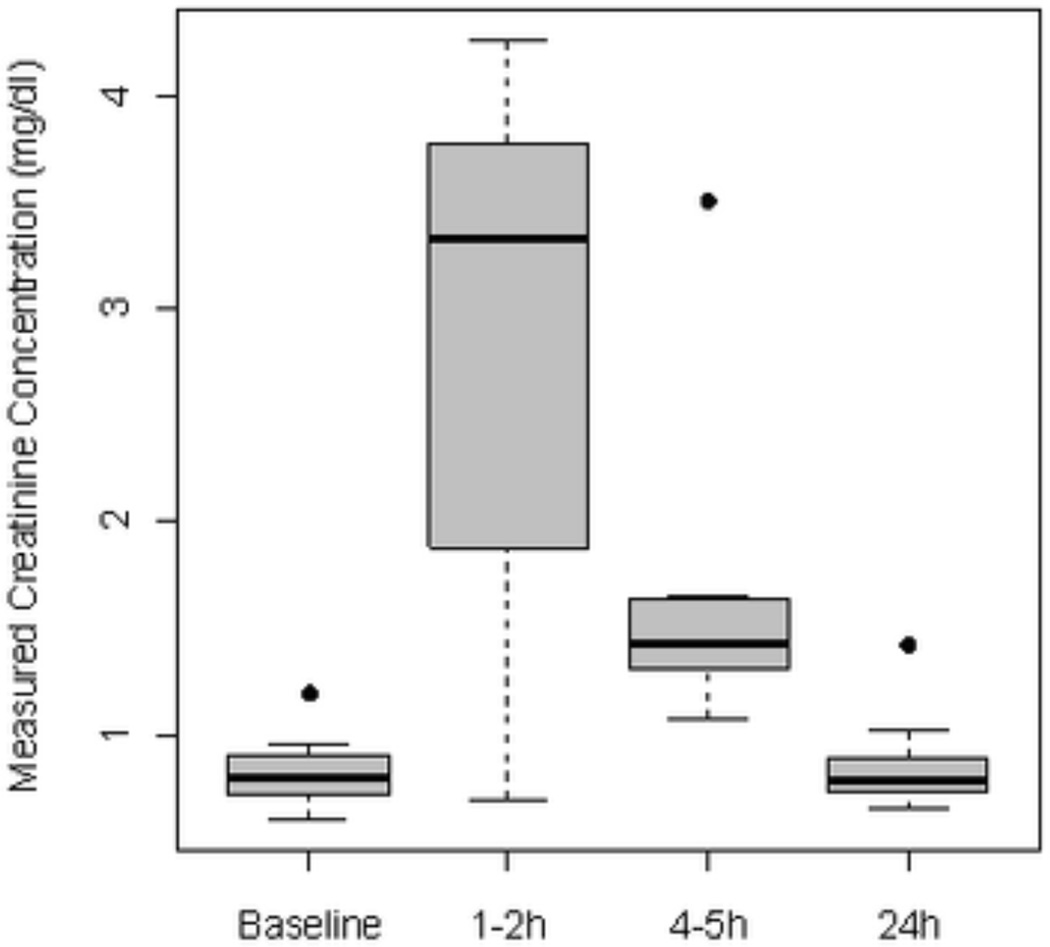
Based on these incidental findings, increased monitoring of serum chemistries for all subsequent study subjects was adopted. The global test indicated evidence of temporal changes in measured creatinine concentration (p=.030). For the majority of patients, a 3–4 fold asymptomatic rise in the serum creatinine was measured within the first 4–5 hours after 5-ALA administration. Differences between baseline and subsequent timepoints achieved statistical significance at both 1–2 hours (p = 0.039) and at the 4–5 h time point (Figure 1, p = 0.008). Serum creatinine levels normalized by 24 hours (p=.444 for the difference with baseline) with no evidence of renal insufficiency or elevated BUN at any point during the study. There was no suggestion that the elevated creatinine was associated with an increased risk of mucositis within the illuminated field as only grade 2–3 mucositis and no grade 4 or greater mucositis was observed.
Figure 1. Box plots of changes in serum creatinine over time, as measured by the Jaffe assay, in patients administered 60 mg/kg of 5-ALA orally (n=11).
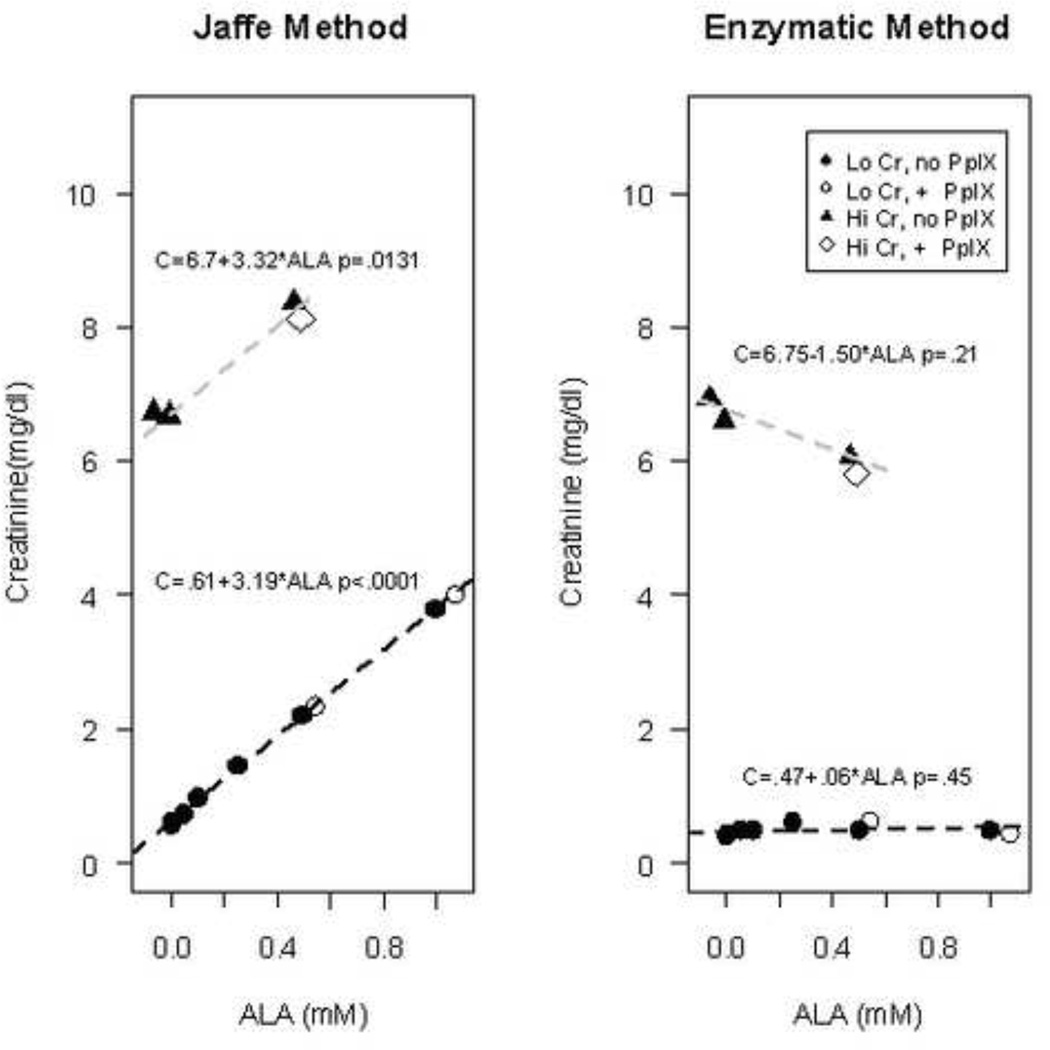
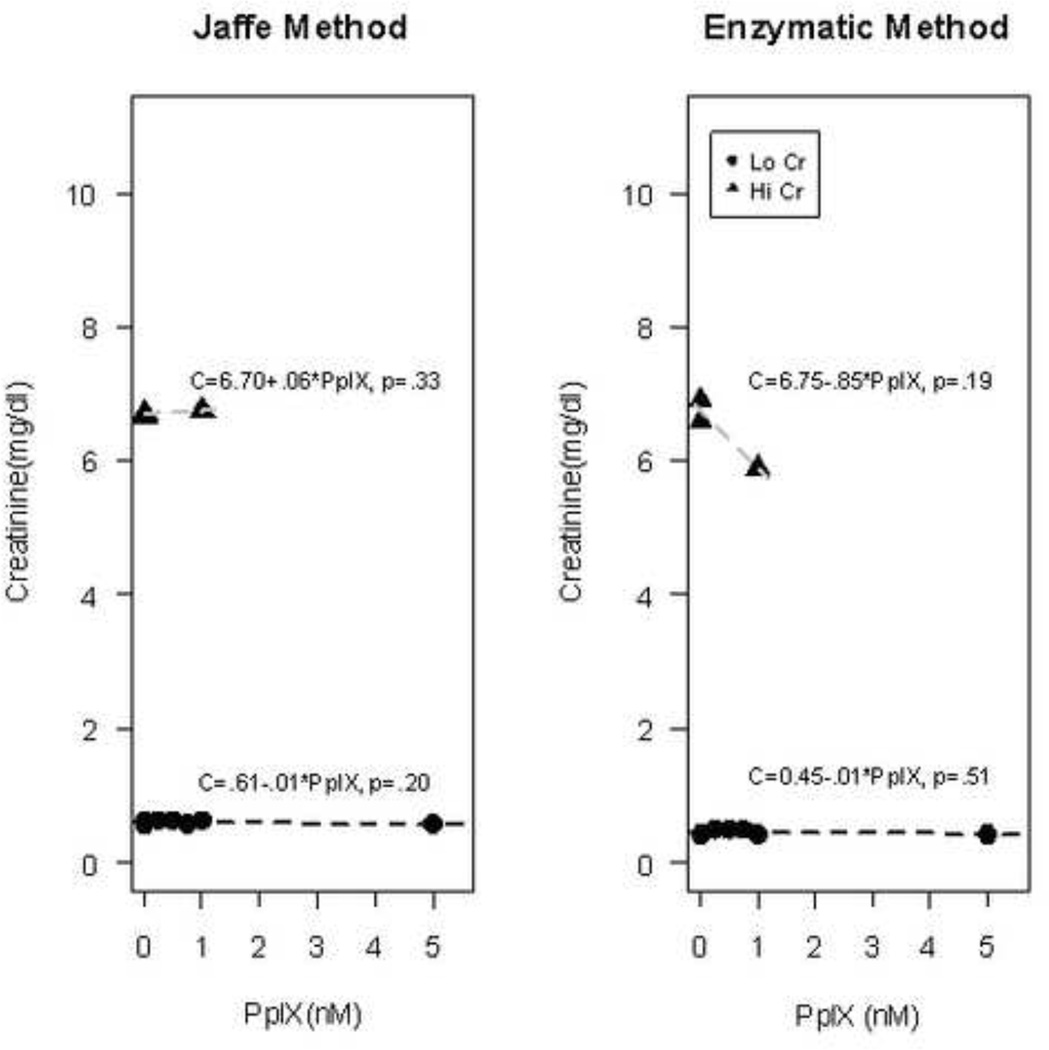
Given that these findings of elevated creatinine were clinically isolated from other measures of renal toxicity, the potential for aberrant results from the assay was considered. Samples of de-identified human serum with low creatinine (Lo Cr) levels (< 0.64 units) were spiked with increasing concentrations of 5-ALA (50 µM – 1 mM) and / or PpIX (250 nm – 5 µM) and assayed for creatinine using both the modified Jaffe method and an alternative enzymatic assay. Similarly, blood samples with pre-existing high creatininine (Hi Cr) levels (>4) were spiked with ALA (0.5 mM) and / or 1 µM PpIX ALA had a concentration-dependent effect on creatinine levels as measured by the Jaffe, but not the enzymatic method (Figure 2). For the Jaffe method, the measured creatinine increased significantly (p<.0001) by 3.18 mg/dl (3.08, 3.30) per 1 mM increase in ALA in the Lo Cr samples and similarly by 3.30 mg/dl (2.44, 4.20, p=.013) per 1 mM increase in ALA in the Hi Cr samples, with the wide confidence interval reflecting the small number of samples in the high creatinine group. For both the Lo Cr and the Hi Cr samples, the measured concentration of creatinine in samples with added PpIX was predicted well by the regression line based on the samples without supplemented PpIX. In contrast, measured creatinine was not affected by the addition of 5-ALA when the enzymatic method was employed. PpIX did not have an effect on creatinine levels as measured by either method (Figure 3).
Figure 2. Effect of ALA on measured creatinine concentration for the Jaffe and Enzymatic methods.
Human blood with pre-existing low creatinine (Lo Cr) levels (<0.64 mg/dL) or high creatinine (Hi Cr) levels (>4 mg/dL) were spiked with ALA (0.05 – 1 mM) and, in the indicated samples, PpIX (1–5 µM). Controls at 0 mM include blood samples with no exogeneous additives, as well as those with the ALA and PpIX vehicles (0.5% DMSO, 0.2% normal saline) alone. Lines shown are for the separate regressions of estimated creatinine concentration in samples spiked only with ALA in the Lo Cr (closed circles) or Hi Cr (closed triangles) groups. The resulting regression equations are given, along with p-values for the test of the null hypothesis that ALA has no effect on measured creatinine concentration; only the Jaffe method showed a significant effect of ALA on measured creatinine concentration. Samples in which PpIX was additionally added (indicated by open symbols) are closely positioned along the regression lines, which indicates that PpIX had minimal effect on the measured creatinine levels.
Figure 3. Effect of PpIX on measured creatinine concentration for the Jaffe and Enzymatic methods.
Human blood with pre-existing low creatinine (Lo Cr) levels (<0.64 mg/dL) or high creatinine (Hi Cr) levels (>4 mg/dL) were spiked with PpIX (0.25–5 µM). Controls at 0 mM include blood samples with no exogeneous additives, as well as those with the ALA and PpIX vehicles (0.5% DMSO, 0.2% normal saline) alone. Lines shown are for the separate regressions of estimated creatinine concentration in samples with Lo Cr (circles) or Hi Cr (triangles) groups. The resulting regression equations are given, along with p-values for the test of the null hypothesis that PpIX has no effect on measured creatinine concentration; a significant effect of PpIX on measured creatinine concentration was not detected under any conditions.
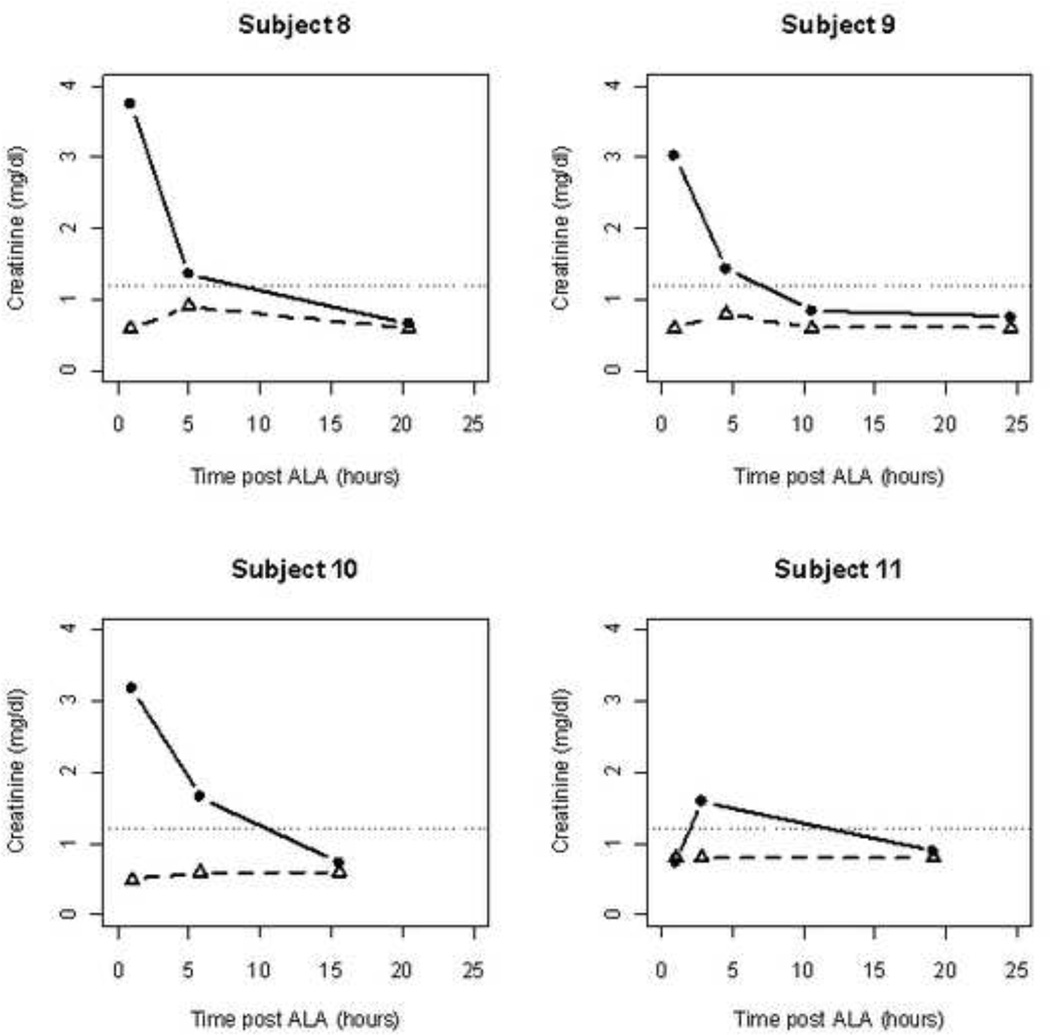
For subjects 8–11, serum creatinine was prospectively evaluated by both the Jaffe and the enzymatic methods. A significant elevation in the creatinine levels were observed with the Jaffe method demonstrating similar asymptomatic creatinine elevations in the first 4–5 hours following ALA administration (Figure 4). However there was no evidence using the enzymatic approach of elevation in creatinine.
Figure 4. Plots of the creatinine concentration measured using either the Jaffe (closed circles) or the enzymatic assay (open triangles).
The dotted line shows the level of creatinine generally considered to be the upper level of the normal range (1.2 mg/dl). While all patients showed at least one elevated level when measured using the Jaffe method, creatinine measured using the enzymatic method was remarkably stable and consistently within the normal range. Mean (upper 95% CI) values of creatinine for the four patients in Figure 4 were 057(0.66), 0.65(0.77), 0.70(0.99), and 0.8(0.97) none of which show any evidence of impaired kidney function.
IV. Discussion
Toxicities from the systemic administration of 5-ALA in humans originally observed by Regula et. al.10 and later described by others11 include transaminitis and mild nausea and vomiting. The former appeared to be dose-related increasing in incidence over a dose of 60 mg/kg. No other abnormalities in serum chemistries have been reported including creatinine which was measured by Webber as early as 12 hours after 30–60 mg/kg 5-ALA11.
The pharmacokinetics of oral and intravenous 5-ALA administration (200 mg/kg) was first described by van den Boogert et. al. in WAG/Rij rats12. A transient rise in serum creatinine was measured as early as 1 hour and peaked at 1 hours following 5-ALA with near and complete resolution by 4 hours and 24 hours, respectively12. Interestingly, these kinetics are almost identical to that observed in our study subjects. After oral administration of ALA, the kinetics of its renal accumulation was also consistent with the kinetics of creatinine elevation with peak accumulation seen at 1–2 hours, followed by rapid decline at 4–6 hours and clearance by 24 hours. In contrast, renal accumulation of PpIX was more gradual and continued to peak up to 12 hours. As the creatinine elevation appears to mimic that of 5-ALA, the suspicion is that our human observations are related to the accumulation of 5-ALA rather than PpIX.
Interferences with measuring creatinine using the Jaffe method are well documented13–16. The basis for the formation of the chromogenic complex in the Jaffe reaction is believed to be the relatively non-specific reaction of the carbonyl group in creatinine with picric acid (13). As such, numerous biologically relevant compounds with similar carbonyl groups have been shown to give falsely elevated creatinine values with this method. These include glucose, ketones, acetoacetate, and cephalosporins, among others13–16. The strength of this interference has been shown to increase with the presence of nitrogen or aromatic groups adjacent to the carbonyl group15. Of note, the compound 5-ALA contains a ketone group with a neighboring nitrogen. This potentially implicates 5-ALA as a newly identified compound which may give falsely elevated creatinine results with the Jaffe method. In agreement, the data of the present report find that the ex vivo addition of ALA to blood samples leads to false increases in measured creatinine levels.
Among the first 7 subjects treated, the kinetics of the rise and fall of the patients’ serum creatinine values over a 24-hour period point to an analytical interference rather than a true physiologic rise in creatinine. To elucidate whether or not ALA also induces a true increase in creatinine, blood samples from patients 8–11 were analyzed by both the Jaffe and enzymatic methods. As found in the majority of the 7 earlier subjects, subjects 8–11 demonstrated significant elevations in creatinine measured by the Jaffe assay. In contrast, the enzymatic method did not detect any significant elevations in creatinine among these patients, and thus suggests that ALA is not truly affecting kidney function.
V. Conclusion
In summary, we report that the Jaffe method, but not an enzymatic assay, finds a significant transient elevation in serum creatinine after oral administration of ALA. The kinetics observed are consistent with observations seen in animal studies and suggest that this may be related to serum and not renal accumulation of 5-ALA. In the limited set of patients examined thus far, the clinical significance of this appears to be inconsequential with no findings that would suggest an increased risk of treatment-related toxicities. We suspect that the elevated creatinine represents a false elevation due to competition by the ALA chromophore in the commonly used Jaffe reaction.
Table 1.
Elevated serum creatinine (Jaffe method) observed following 5-aminolevulinic acid (60 mg/kg)
| Subject | Light Dose (J/cm2)3 |
Creatinine 1 | Maximum mucositis grade2 |
|||
|---|---|---|---|---|---|---|
| Baseline | 1–2 h | 4–5 h | 24 h | |||
| 1 | 50 continuous |
1.2 | 1.42 | 3 | ||
| 2 | 50 continuous |
0.87 | 0.82 | 3 | ||
| 3 | 50 continuous |
0.74 | 1.08 | 0.78 | 2 | |
| 4 | 50 fractionated |
0.70 | 3.48 | 1.28 | 0.65 | 3 |
| 5 | 50 fractionated |
0.95 | 0.69 | 3.5 | 1.02 | 3 |
| 6 | 50 fractionated |
0.63 | 3.8 | Not available |
0.74 | 2 |
| 7 | 50 fractionated |
0.86 | 4.26 | 1.44 | 0.86 | 2 |
| 8 | 100 continous |
0.76 | 3.01 | 1.43 | 0.75 | 2 |
| 9 | 100 fractionated |
0.61 | 3.74 | 1.35 | 0.66 | 3 |
| 10 | 100 fractionated |
0.80 | 3.19 | 1.66 | 0.74 | 3 |
| 11 | 100 continous |
0.94 | 0.74 | 1.60 | 0.90 | 2 |
mg/dL (normal range: 0.64–1.27)
NCI Common Toxicity Criteria version 3.0 within the 4 weeks of follow-up evaluation.
Light dose was administered either continuously or interrupted for a time interval of 90–180 seconds.
Table 2.
Measured Creatinine Levels in Human Blood Samples with Exogeneously added ALA or PpIX
| Pooled serum with low creatinine (<0.64) | ||
|---|---|---|
| Supplement | Jaffe Method | Enzymatic Reaction |
| No Supplements | 0.59 | 0.4 |
| 0.5% DMSO, 0.2% NS | 0.58 | 0.4 |
| ALA | ||
| 0.05 mM ALA | 0.74 | 0.5 |
| 0.10 mM ALA | 0.98 | 0.5 |
| 0.25 mM ALA | 1.46 | 0.6 |
| 0.50 mM ALA | 2.21 | 0.5 |
| 1.0 mM ALA | 3.78 | 0.5 |
| PpIX | ||
| 0.25 μM PpIX | 0.62 | 0.5 |
| 0.50 μM PpIX | 0.63 | 0.5 |
| 0.75 μM PpIX | 0.58 | 0.5 |
| 1.0 μM PpIX | 0.62 | 0.4 |
| 5.0 μM PpIX | 0.56 | 0.4 |
| ALA + PpIX | ||
| 0.50 mM ALA + 1.0 μM PpIX | 2.19 | 0.6 |
| 1.0 mM ALA + 5.0 μM PpIX | 3.77 | 0.4 |
| Pooled serum with high creatinine (>4) | ||
| Supplement | Jaffe Method | Enzymatic Reaction |
| No Supplements | 6.68 | 6.6 |
| 0.5% DMSO, 0.2% NS | 6.72 | 6.9 |
| 0.50 mM ALA | 8.36 | 6 |
| 1.0 μM PpIX | 6.76 | 5.9 |
| 0.50 mM ALA + 1.0 μM PpIX | 8.24 | 5.9 |
Acknowledgments
This study was supported in part by the National Institutes of Health (R01-CA-129554) and American Cancer Society (IRG-78-002-28). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Cancer Institute.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest: None
Disclosure: 5-aminolevulinic acid provided by DUSA Pharmaceuticals.
References
- 1.Lang K, Schulte KW, Ruzicka T, Fritsch C. Aminolevulinic acid (Levulan) in photodynamic therapy of actinic keratoses. Skin therapy letter. 2001;6(10):1–2. 5. [PubMed] [Google Scholar]
- 2.Ackroyd R, Brown N, Vernon D, et al. 5-Aminolevulinic acid photosensitization of dysplastic Barrett's esophagus: a pharmacokinetic study. Photochemistry and photobiology. 1999;70(4):656–662. [PubMed] [Google Scholar]
- 3.Collaud S, Juzeniene A, Moan J, Lange N. On the selectivity of 5-aminolevulinic acid-induced protoporphyrin IX formation. Current medicinal chemistry. 2004;4(3):301–316. doi: 10.2174/1568011043352984. [DOI] [PubMed] [Google Scholar]
- 4.Krammer B, Plaetzer K. ALA and its clinical impact, from bench to bedside. Photochem Photobiol Sci. 2008;7(3):283–289. doi: 10.1039/b712847a. [DOI] [PubMed] [Google Scholar]
- 5.Finlay JC, Conover DL, Hull EL, Foster TH. Porphyrin bleaching and PDT-induced spectral changes are irradiance dependent in ALA-sensitized normal rat skin in vivo. Photochemistry and photobiology. 2001;73(1):54–63. doi: 10.1562/0031-8655(2001)073<0054:pbapis>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 6.Webber J, Kessel D, Fromm D. Plasma levels of protoporphyrin IX in humans after oral administration of 5-aminolevulinic acid. Journal of photochemistry and photobiology. 1997;37(1–2):151–153. doi: 10.1016/s1011-1344(96)07348-4. [DOI] [PubMed] [Google Scholar]
- 7.Beckman Coulter synchron system chemistry information sheet. Accessed at http://beckmancoulter.com/vsearch/view.asp?piMode=true&serverSpec=svfulwebsrch:9920&querytext=synchron+system+CREA+information+sheet&OrigQuery=&QueryParser=Internet_Basic&k2dockey=http%3A%2F%2Fwww%2Ebeckmancoulter%2Ecom%2Fcustomersupport%2FIFU%2Fcis%2F389736%2FAE%2FEN%5FCREA%2Edoc%40BC%5FWEB&logTitle=&DirectURL=http%3A%2F%2Fwww%2Ebeckmancoulter%2Ecom%2Fcustomersupport%2FIFU%2Fcis%2F389736%2FAE%2FEN%5FCREA%2Edoc&dtype=1#hit0. [Google Scholar]
- 8.Nova Biomedical Critical Care Xpress Procedure Manual: Creatinine(Creat) Concentration. 2010 Apr 27; [Google Scholar]
- 9.A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2009. [Google Scholar]
- 10.Regula J, MacRobert AJ, Gorchein A, et al. Photosensitisation and photodynamic therapy of oesophageal, duodenal, and colorectal tumours using 5 aminolaevulinic acid induced protoporphyrin IX--a pilot study. Gut. 1995;36(1):67–75. doi: 10.1136/gut.36.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Webber J, Kessel D, Fromm D. Side effects and photosensitization of human tissues after aminolevulinic acid. The Journal of surgical research. 1997;68(1):31–37. doi: 10.1006/jsre.1997.5004. [DOI] [PubMed] [Google Scholar]
- 12.van den Boogert J, van Hillegersberg R, de Rooij FW, et al. 5-Aminolaevulinic acid-induced protoporphyrin IX accumulation in tissues: pharmacokinetics after oral or intravenous administration. Journal of photochemistry and photobiology. 1998;44(1):29–38. doi: 10.1016/s1011-1344(98)00102-x. [DOI] [PubMed] [Google Scholar]
- 13.Koumantakis G, Wyndham L. Fluorescein interference with urinary creatinine and protein measurements. Clinical chemistry. 1991;37(10 Pt 1):1799. [PubMed] [Google Scholar]
- 14.Da Rin G, Amici G, Virga G, Bardin C, Calzavara P, Bocci C. Correction of glucose concentration interference on Jaffe kinetic creatinine assay in peritoneal dialysis. American journal of nephrology. 1995;15(6):480–487. doi: 10.1159/000168890. [DOI] [PubMed] [Google Scholar]
- 15.Kroll MH, Roach NA, Poe B, Elin RJ. Mechanism of interference with the Jaffe reaction for creatinine. Clinical chemistry. 1987;33(7):1129–1132. [PubMed] [Google Scholar]
- 16.Saenger AK, Lockwood C, Snozek CL, et al. Catecholamine interference in enzymatic creatinine assays. Clinical chemistry. 2009;55(9):1732–1736. doi: 10.1373/clinchem.2009.127373. [DOI] [PubMed] [Google Scholar]