Abstract
Background
Plant fungi (e.g., Pellicularia sasakii, Gibberella zeae, Fusarium oxysporum, and Cytospora mandshurica and Phytophthora infestans) and bacteria (e.g., Ralstonia solanacearum) are extremely difficult to manage in agricultural production. The high incidence of plant mortality and the lack of effective control methods make P. sasakii and R. solanacearum two of the world’s most destructive plant pathogens. Pathogenic fungi and bacteria are responsible for billions of dollars in economic losses worldwide each year. Thus, we designed an active amide structure and synthesized a series of novel amide derivatives containing a triazole moiety to discover new bioactive molecules and pesticides that can act against fungi and bacteria.
Results
A series of amide derivatives containing a triazole moiety were synthesized. All the obtained compounds were characterized through proton and carbon nuclear magnetic resonance spectroscopy, infrared spectroscopy, and elemental analysis. Preliminary antifungal activity test showed that some of the synthesized compounds exhibited moderate antifungal activity against P. sasakii, G. azeae, F. oxysporum, C. mandshurica, and P. infestans at 50 mg/L. Compound 4u displayed more potent antifungal activity against P. sasakii and G. azeae than hymexazol. Preliminary antibacterial activity results showed that some of the synthesized compounds exhibited high anti-bacterial activity against R. solanacearum at 200 mg/L. Compounds 4m and 4q displayed high antibacterial activity against R. solanacearum, with 71% and 65% inhibitory rates, respectively.
Conclusions
A series of novel amide derivatives containing 1,2,4-triazole moiety were synthesized through the reaction of intermediate 3 with different acyl chlorides and anhydrous potassium carbonates in anhydrous tetrahydrofuran at 50°C, using 2,4-dichloroacetophenoneas as a starting material. The title compounds exhibited high inhibitory effects against P. sasakii, R. solanacearum, and G. azeae.
Background
1,2,4-Triazole, an important class of heterocyclic rings, has attracted increasing attention due to their broad activities, such as fungicidal [1,2], insecticidal [3,4], herbicidal [5,6], and bactericidal [7,8]. It may also serve as a plant growth regulatory agent [9] and has excellent potential in the pesticide field. Since the discovery of triadimefon by Bayer in 1976, 1,2,4-triazole has been used as fungicide for approximately 30 years. It quickly gained a significant importance in the protection of various crops, representing a significant progress in the chemical control of fungal diseases. With the increasing number of triazole derivatives, several compound containing tebuconazole, propiconazole, and metconazole have been developed and commercialized, respectively (Figure 1).
Figure 1.
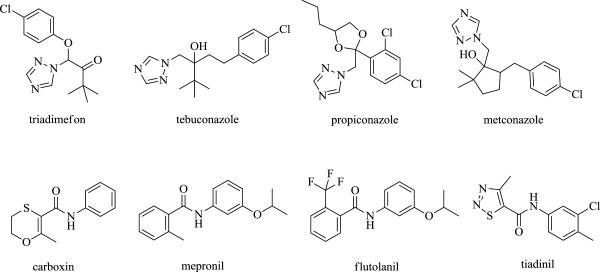
Commercialized fungicides containing 1,2,4-triazole or amide substructures.
Since the first synthesis of carboxin by Schmeling and Kulkain in 1966 [10], amide fungicides have also been used for controlling plant diseases for more than 40 years. Amide derivatives have become a research hot spot in the development of pesticides because of their high-efficiency active features and broad spectrum bioactivities, such as antifungal [11,12], insecticidal [13], and herbicidal [14]. Currently, some amide derivatives have been developed and commercialized as pesticides. Mepronil, flutolanil, and tiadinil are known for their ability to protect certain plants from severe diseases and pests (Figure 1). In our recent publications [15,16], several pyrazole amide derivatives containing a hydrazone moiety have been synthesized and tested for their antifungal activity. The synthesized compounds exhibited antifungal activity against Fusarium oxysporum and Cytospora mandshurica, with inhibitory rates ranging from 40.82% to 50.32%. In addition, some hydrazone derivatives containing a pyridine moiety possessed high antibacterial activity against Ralstonia Solanacearum[16].1-(2,4-Dichlorophenyl)-3-aryl-2-(1H-1,2,4-triazol-1-yl) prop-2-en-1-one derivatives have been synthesized using aldol condensation between 1-(2,4-dichlorophenyl)-2-(1H-1,2,4-triazol-1-yl) ethan one and an aryl aldehyde. The better compound showed an antifungal activity level similar to that displayed by hymexazol against Gibberell azeae, F. oxysporum, and C. mandshurica[17].
The resistance of pathogens toward currently available drug therapies is rapidly becoming a major worldwide problem. Thus, the design of new compounds for resistant fungi and bacteria has become one of the most important areas of antibacterial research to date. Plant fungi (e.g., P. sasakii, G. azeae, F. oxysporum, C. mandshurica, and P. infestans) and bacteria (e.g., R. solanacearum) are extremely difficult to control in agricultural production. Pathogenic fungi and bacteria are responsible for billions of dollars in economic losses worldwide each year. In addition, the application of traditional pesticides is not effective and causes high residue level or negative impact on the environment. Therefore, searching for new antifungal and antibacterial agents remains a daunting task in pesticide science. In current study, we combined the active structure of amide and 1, 2, 4-triazole to design and synthesize a series of novel amide derivatives containing a triazole moiety to discover new bioactive molecules and pesticides that can act against fungi and bacteria. Using 2,4-dichloroacetophenone as a starting material, twenty-two novel analogs of amide containing 1,2,4-triazole were synthesized. All the compounds were unequivocally characterized by infrared (IR) spectroscopy, proton and carbon nuclear magnetic resonance spectroscopy (1H NMR and13C NMR, respectively), and elemental analysis. The biological activity of the compounds against G. azeae, F. oxysporum, C. mandshurica, P. sasakii, and P. infestans were tested. The results showed that most of the synthesized compounds exhibited antifungal activity against G. azeae, F. oxysporum, C. mandshurica, P. sasakii, and P. infestans at 50 mg/L and antibacterial activity against R. solanacearum at 200 mg/L. Compounds 3e and 3g showed high antibacterial activity at 200 mg/L. According to the results of bioassay, compound 4u displayed higher potent antifungal activity against P. sasakii and G. azeae than hymexazol. In addition, compounds 4m and 4q displayed high antibacterial activity against R. solanacearum at 200 mg/L, with 71% and 65% inhibitory rates, respectively. To the best of our knowledge, this study is the first to report on the antibacterial activity of amide derivatives containing a 1,2,4-triazole moiety.
Results and discussion
Synthesis
The synthetic route to the title compounds is demonstrated in Additional file 1. Using readily available starting materials, intermediates 1 and 2 were prepared following a previously described procedure [17]. The corresponding acyl chloride was prepared by refluxing acid in thionylchloride for 8 h, and then the solution was diluted with dry dichloromethane at 50°C. The aldol reaction of intermediate 2 with 4-aminobenzaldehyde in dry tetrahydrofuran (THF) using piperidine as a catalyst can proceed readily at 60°C to obtain intermediate 3. Subsequent treatment of intermediate 3 with acyl chloride and potassium carbonate in dry THF solvent at ambient temperature afforded the desired compounds (4a to 4v) in 40% to 70% yields. The synthesis of compound 4i was carried out under different conditions to optimize the reaction conditions for the preparation of the title compounds. The effects of different solvents, reaction times, acid binding agents, and reaction temperatures are summarized in Table 1. The yields of compound 4i were 15.3%, 22.1%, and 40.0% when toluene, acetonitrile, and dichloromethane were used as solvents, respectively (Table 1, Entries 2 to 4). Meanwhile, the yield reached up to 73.8% when the reaction mixture was at 50°C for 8 h in THF (Table 1, Entry 1). The yields of compound 4i were 33.6%, 55.8.1%, and 73.8% when triethylamine, pyridine, and potassium carbonate were used as acid binding reagents in the reaction, respectively (Table 1, Entries 5 to 7). However, no significant improvement (76.4%, Entry 9) was observed when the reaction time was prolonged from 8 h to 10 h (73.8%, Entry 1).The yield was lower (60.1% after 8 h, Entry 10; 62.2% after10 h, Entry 11) at 25°C than that of 50°C. Hence, the optimum condition was selected in THF with potassium carbonate at 50°C for 8 h. The synthetic route in Scheme 1 has several advantages, including simple procedures, short reaction times, moderate yields, and mild conditions (room temperature).
Table 1.
Yields of compound 4i at different reaction conditions
| Entry | Solvent | Time/h | Acid binding agent | Temperature/°C | Yield/% |
|---|---|---|---|---|---|
| 1 |
Tetrahydrofuran |
8 |
Potassium carbonate |
50 |
73.8 |
| 2 |
Toluene |
8 |
Potassium carbonate |
50 |
15.3 |
| 3 |
Acetonitrile |
8 |
Potassium carbonate |
50 |
22.1 |
| 4 |
Dichloromethane |
8 |
Potassium carbonate |
50 |
40.0 |
| 5 |
Tetrahydrofuran |
8 |
Triethylamine |
50 |
33.6 |
| 6 |
Tetrahydrofuran |
8 |
Pyridine |
50 |
55.4 |
| 7 |
Tetrahydrofuran |
4 |
Potassium carbonate |
50 |
62.5 |
| 8 |
Tetrahydrofuran |
6 |
Potassium carbonate |
50 |
67.8 |
| 9 |
Tetrahydrofuran |
10 |
Potassium carbonate |
50 |
76.4 |
| 10 |
Tetrahydrofuran |
8 |
Potassium carbonate |
25 |
60.1 |
| 11 | Tetrahydrofuran | 10 | Potassium carbonate | 25 | 62.2 |
Additional file 2 provides the structure, yield, and elemental analysis data for the title compounds.
The structures of the synthesized compounds were confirmed by elemental analysis and 1H-NMR, 13C-NMR, and IR spectroscopy. The IR spectral data of compounds 4a to 4v showed characteristic absorption bands of NH at 3088 cm-1to 3444 cm-1. The absorption bands of the carbonyl and C=C groups of α, β-unsaturated carbonyl skeleton appeared at 1690 cm-1 to 1630 cm-1 and 1530 cm-1 to 1560 cm-1, respectively. In the 1H-NMR spectra of the title compounds, most phenyl protons showed multiple at 6.87 ppm to 8.38 ppm. Notably, the phenyl protons of compound 4p at 9.01 and 9.12 ppm appeared as a singlet because of the existence of two nitro groups in the 3,5-position of the benzene ring, which led to its chemical shift moving to a lower field. The compounds showed the NH proton at 10.53 ppm to 11.07 ppm as a broad singlet. The two protons of the triazole ring appeared at 8.07 ppm to 8.74 ppm and 7.97 ppm to 8.35 ppm. The Ar-OH proton appeared as a broad singlet at 12.07 ppm to 11.44 ppm, and the methyl (Ar-CH3) proton signals were observed as a singlet near 2.18 ppm to 2.34 ppm.
Biological activity and structure-activity relationship (SAR)
Antifungal activity
The antifungal bioassay results are shown in Table 2. Hymexazol, one of the commercial fungicides for controlling G. azeae, F. oxysporum, C. mandshurica, P. sasakii, and P. infestans, was used as the positive control. These newly synthesized amide derivatives containing a triazole moiety exhibited low to high antifungal activities against the tested fungi at 50 mg/L. Compounds 4i, 4j, 4k, and 4u inhibited the growth of G. azeae at 40.06%, 41.22%, 46.44%, 46.01%, and 58.90%, respectively. The activities of compounds 4i, 4j, 4k, 4q, 4r, 4u, and 4v against F. oxysporum were 33.12%, 32.18%, 37.02%, 33.74%, 35.23%, 52.11%, and 30.68%, respectively. And compounds 4i, 4j, 4k, 4q, 4r, and 4u showed 29.41%, 30.11%, 40.01%, 30.65%, 36.77%, 48.61%, and 29.55% activities against C. mandshurica, respectively; compounds 4i, 4j, 4k, 4q, and 4u displayed activities against P. sasakii at 50.06%, 50.22%, 39.68%, 51.25%, and 60.01%, respectively. Moreover, inhibitory rates of compounds 4i, 4j, 4k, 4q, 4r, and 4u on P. infestans were 22.52%, 22.18%, 18.82%, 19.04%, 10.33%, and 47.31%, respectively. The preliminary SAR based on the activity against G. azeae showed that the substituent group at the 2-position of the benzene ring had an important effect on the antifungal activity of the title compounds. The antifungal activity of the designed compounds decreased when hydroxyl was substituted at the 2-position of the benzene ring, with inhibitory rates ranging from 5.20% to 35.01%. The antifungal activity of compound 4i (inhibitory rate: 40.44%) without any substituent on the phenyl ring was higher than that of 4a (inhibitory rate: 29.04%) with hydroxyl at the 2-position of the benzene ring. The variation in substituent on the phenyl ring also caused the different antifungal activities of the title compounds, with inhibitory rates ranging from 0% to 46.44%. For instance, the inhibitory rate of compound 4j with 2,4-di-fluoro substituent on the phenyl ring was 41.22%, whereas that of compound 4m with 2,4-di-chloro substituent on the phenyl ring was 31.67%. Furthermore, the compounds with the same substituent but at different positions on the phenyl ring exhibited different antifungal activities. For example, compound 4k with chorine at the 2-position of the benzene ring possessed high inhibitory activity against G. azeae, whereas compound 4l with chorine at the 4-position of the benzene ring displayed moderate activity. Moreover, when the benzene ring was replaced with a furan ring in the title compounds, compound 4u showed potent antifungal activity, with inhibitory rates against G. azeae, F. oxysporum, C. mandshurica, and P. sasakii ranging from 48.61% to 60.01%. Similar inhibitory rates were exhibited by hymexazol, with 55.54%, 56.12%, 49.61%, and 51.21% against G. azeae, F. oxysporum, C. mandshurica, and P. sasakii at 50 mg/L, respectively.
Table 2.
Antifungal activity of title compounds 4a to 4v at a concentration of 50 mg/L
|
Compound |
Inhibition ratea(%) |
||||
|---|---|---|---|---|---|
| G. zeae | F.oxysporum | C.mandshurica | P.sasakii | P. infestans | |
|
4a |
29.02±0.96 |
22.91±1.26 |
24.45±0.74 |
17.12±0.76 |
13.96±1.09 |
|
4b |
35.01±0.82 |
31.26±0.84 |
13.93±1.86 |
18.41±0.75 |
10.56±0.79 |
|
4c |
34.40±0.90 |
5.88±0.92 |
21.55±1.32 |
12.30±0.91 |
14.88±0.89 |
|
4d |
22.39±0.80 |
8.66±0.91 |
16.71±0.82 |
12.33±0.94 |
15.61±1.41 |
|
4e |
17.35±1.01 |
5.26±0.84 |
8.97±0.72 |
11.55±1.31 |
15.26±0.83 |
|
4f |
29.38±0.91 |
26.38±0.88 |
20.38±0.61 |
13.78±0.92 |
16.33±0.68 |
|
4g |
5.90±1.02 |
7.84±1.12 |
12.01±0.88 |
10.55±1.12 |
17.82±0.99 |
|
4h |
17.82±1.22 |
25.75±0.96 |
21.56±0.91 |
17.52±0.72 |
11.53±0.96 |
|
4i |
40.06±0.87 |
33.12±0.86 |
29.41±0.83 |
50.06±0.81 |
22.52±0.96 |
|
4j |
41.22±1.00 |
32.18±0.89 |
30.11±1.33 |
50.22±1.10 |
22.18±0.80 |
|
4k |
46.44±0.93 |
37.02±0.79 |
40.01±1.17 |
39.68±0.77 |
18.82±0.90 |
|
4l |
32.66±0.80 |
10.12±0.79 |
18.69±0.93 |
36.61±0.91 |
10.32±0.89 |
|
4m |
31.67±0.79 |
15.68±0.82 |
19.00±1.34 |
28.68±0.99 |
15.64±1.12 |
|
4n |
7.80±1.36 |
4.65±0.76 |
24.36±1.02 |
10.31±0.79 |
7.14±0.86 |
|
4o |
38.64±0.95 |
6.52±0.99 |
27.36±1.44 |
30.14±0.85 |
13.02±1.09 |
|
4p |
0 |
0 |
12.69±1.00 |
18.12±0.65 |
5.10±0.89 |
|
4q |
30.28±0.92 |
33.74±0.98 |
30.65±0.95 |
51.25±0.87 |
19.04±0.88 |
|
4r |
39.64±0.88 |
35.23±1.32 |
36.77±0.75 |
11.32±0.78 |
10.33±1.02 |
|
4s |
22.90±1.25 |
24.36±0.91 |
26.69±0.89 |
20.84±1.11 |
14.33±0.81 |
|
4t |
18.69±1.20 |
20.13±0.72 |
15.36±0.90 |
19.61±0.80 |
10.13±0.72 |
|
4u |
58.90±0.64 |
52.11±1.44 |
48.61±1.03 |
60.10±0.86 |
47.31±1.14 |
|
4v |
35.12±0.87 |
30.68±0.79 |
29.55±0.94 |
28.12±0.79 |
16.88±0.91 |
| Hymexazolb | 55.54±3.90 | 56.12±4.10 | 49.61±7.84 | 51.21±5.96 | 68.22±2.41 |
aAverage of five replicates, bThe commercial agricultural fungicide hymexazol was used for activity comparison.
Antibacterial activity
The results of antibacterial bioassay are given in Table 3. Kocide®3000, one of the proven commercial agents for controlling R. solanacearum, was used as a reference for bactericides. Most of the prepared compounds showed low to high antibacterial activities against R. solanacearum at 200 mg/L. Compounds 4c, 4n, and 4s displayed higher activities than the other compounds at 200 mg/L, reaching 55%, 71%, and 65% inhibitory rates, respectively. The free hydroxyl group plays an important role in the antibacterial activity. Compound 4b with hydroxyl at the 2-position and chlorine at the 4-position of the benzene ring displayed higher antibacterial activity against R. solanacearum than that of compound 4l with chlorine at the 4-position of the benzene ring. Substituting a methoxy at the 2-position of the benzene ring also improved the antibacterial activity. For example, after introducing a methoxy group into the 2-position of the benzene ring, the inhibitory rate of 4q (R=2-methoxyphenyl) was 65%. In addition, the inhibitory rates of compounds 4a (R=2-hydroxyphenyl), 4s (R=2-fluorophenyl), and 4k (R=2-chlorophenyl) against tobacco bacterial wilts were 0%, 11%, and 24%, respectively. Furthermore, the activity of the compounds with the same position but with different substituents on the phenyl ring exhibited different activities. For example, the inhibitory rate of compound 4n with 2,4-dicloro substituent on the phenyl ring was 71%, whereas that of compound 4k with 2,4-difluro substituent on the phenyl ring was 40%. Unlike antifungal activity, high antibacterial activity was not obtained when the benzene ring was replaced with a heterocyclic ring, i.e., furan ring for compound 4u and 2-pyridine ring for compound 4v. Both compounds 4u and 4v displayed low antibacterial activity.
Table 3.
Antibacterial activity of compounds 4a to 4v against R. solanacearum
|
Compound |
Inhibition rate (%)a |
|
|---|---|---|
| 200 mg/L | 100 mg/L | |
|
4a |
0 |
/ |
|
4b |
55 |
23 |
|
4c |
0 |
/ |
|
4d |
0 |
/ |
|
4e |
30 |
28 |
|
4f |
14 |
6 |
|
4g |
0 |
/ |
|
4h |
17 |
0 |
|
4i |
0 |
/ |
|
4j |
40 |
0 |
|
4k |
24 |
0 |
|
4l |
17 |
10 |
|
4m |
71 |
29 |
|
4n |
11 |
6 |
|
4o |
38 |
5 |
|
4p |
0 |
/ |
|
4q |
65 |
26 |
|
4r |
16 |
6 |
|
4s |
11 |
14 |
|
4t |
29 |
21 |
|
4u |
0 |
/ |
|
4v |
22 |
1 |
| Kocide3000 [Cu(OH)2] | 100.0 | 100.0 |
Experimental
Chemistry
Melting points were determined using a XT-4 binocular microscope (Beijing Tech Instrument Co., Beijing, China) and uncorrected. 1H and 13C-NMR spectra were recorded on a JEOL-ECX 500 NMR spectrometer operating at 500 MHz for 1H-NMR and 125 MHz for 13 C-NMR at room temperature using DMSO-d6 as a solvent and tetramethylsilane as an internal standard. IR spectra were recorded in KBr on an IR Pristige-21 spectrometer (Shimadzu Corporation, Japan). Elemental analysis was performed using an Elemental Vario-III CHN analyzer. Analytical thin layer chromatography was performed on silica gel GF254. Unless otherwise stated, all reagents and reactants were purchased from commercial suppliers and were of analytical grade or chemically pure. All anhydrous solvents were dried and purified according to standard techniques before use. 2-Bromo-1-(2,4-dichlorophenyl) ethanone, intermediate 2, and sodium-1,2,4-triazolide were prepared according to previously reported methods [18,19] and used without further purification (Additional file 3).
Antifungal biological assay
The antifungal activity of all synthesized compounds was tested against five pathogenic fungi, G. azeae, F. oxysporum, C. mandshurica, P. sasakii, and P. infestans, through the poison plate technique [20]. All the compounds were dissolved in dimethyl sulfoxide (DMSO, 10 mL) before mixing with potato dextrose agar (PDA, 90 mL). The compounds were tested at a concentration of 50 mg/L. All fungal species were incubated in PDA at 27 ±1°C for 5 d to obtain new mycelium for antifungal assay. Mycelia dishes approximately 4 mm in diameter were cut from the culture medium. One of the specimens was picked up with a sterilized inoculation needle and then inoculated in the center of the PDA plate aseptically. The inoculated plates were incubated at 27 ±1°C for 5 d. DMSO in sterile distilled water served as the control, whereas hymexazole acted as the positive control. Three replicates were conducted for each treatment. The radial growth of the fungal colonies was measured, and the data were statistically analyzed. The inhibitory effects of the test compounds in vitro against these fungi were calculated as follows:
where C represents the diameter of fungal growth on untreated PDA, T represents the diameter of fungi on treated PDA, and I is the inhibitory rate.
Antibacterial Biological Assay
The antibacterial activities of some title compounds against R. solanacearum were evaluated via the turbidimeter test [21], with Kocide®3000 as the positive control. The compounds were dissolved in 150 μL DMSO and diluted with water containing Tween-20 (0.1%) to generate final concentrations of 200 and 100 mg/L, which were then added to the toxic nutrient broth (NB) liquid medium in 4 mL tubes. Approximately 80 μL NB liquid medium containing R. solanacearum was individually added to the tubes and then shaken at 180 rpm for 48 h at 30°C. The relative inhibitory rate of the circle mycelium compared with a blank assay was calculated as follows:
Where A0 and A1represent the corrected optical density values of the control medium of bacilli and the toxic medium, respectively.
Conclusion
In summary, a series of novel amide derivatives containing a triazole moiety were designed and synthesized through the reaction of intermediate 3 with different acyl chlorides and anhydrous potassium carbonates in THF at room temperature using 2,4-dichloroacetophenone as a starting material. All the prepared compounds were characterized by spectral data (1H-NMR, 13C-NMR, and IR) and elemental analysis. The fungicidal activities in vitro of the compounds against G. azeae, F. oxysporum, C. mandshurica, P. sasakii, and P. infestans were evaluated. The results showed that the title compounds possessed low to high antifungal activities against the tested fungi. Compound 4u displayed high antifungal activity. Furthermore, the antibacterial tests indicated that some of the synthesized compounds also possessed moderate activity against R. solanacearum. Compounds 4b, 4m, and 4q exhibited high inhibitory activity against tobacco bacterial wilts in vitro. The results of preliminary SAR study indicated that the fungicidal activity can be decreased by introduction of hydroxyl at the 2-position of the benzene ring, the compound containing a furan displayed higher antifungal activity against different fungi than that of benzene, and the substituent of 2,4-di-fluoro on the phenyl ring can enhance the activities against G. azeae. However, unlike antifungal activity, the free hydroxyl group at the 2-position of the benzene plays an important role in the antibacterial activity against R. solanacearum, and the compounds containing 2,4-dicloro showed much higher activity than that of 2,4-difluro, and the introduction of heterocyclic ring could decrease the antibacterial activity. Moreover, the methoxy at the 2-position of the benzene ring also improved the antibacterial activity. Further studies are currently underway to establish a definite SAR.
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
The current study is an outcome of the constructive discussion with Profs.BAS, LHJ, DYH, and SY, who offered necessary guidance to RPT and CLM to carry out their synthesis and characterization experiments. Both RPT and CLM were also involved in drafting the manuscript. JY performed the antifungal tests; JW and LHJ carried out the 1H NMR and13C NMR spectral analyses and elemental analysis. BAS and SB were involved in revising the manuscript. All authors read and approved the final manuscript.
Supplementary Material
Synthetic route to target compounds 4a to 4v. Synthetic sequence to the novel amide derivatives containing a triazole moiety from intermediate 3.
Yield and elemental analysis data for title compounds 4a to 4v. Description: Structure, yield, and elemental analysis data for title compounds 4a to 4v.
Experimental details and data of the title compounds 4a to 4v. Experimental procedure, spectroscopic data of intermediate 3, title compounds 4a to 4v, copies of 1H NMR, 13C NMR, and IR spectroscopy.
Contributor Information
Ruping Tang, Email: eziotang@yahoo.cn.
Linhong Jin, Email: fcc.jinlh@gzu.edu.cn.
Chengli Mou, Email: mouchengli_h@163.com.
Juan Yin, Email: yinjuan2009292018@126.com.
Song Bai, Email: basonmail@163.com.
Deyu Hu, Email: fcc.dyhu@gzu.edu.cn.
Jian Wu, Email: jianwu2691@yahoo.com.cn.
Song Yang, Email: fcc.syang@gzu.edu.cn.
Baoan Song, Email: basong@gzu.edu.cn.
Acknowledgements
This study is financially supported by the National Natural Science Foundation of China (No. 20962005) and Key Technologies R&D Program (No.2011BAE06B05-6).
References
- Yu GP, Xu LZ, Yi X, Bi WZ, Zhu Q, Zhai ZW. Synthesis and fungicidal evaluation of 2-arylphenyl ether-3-1H-1,2,4-triazol-1-yl) propan-2-ol derivatives. J Agric Food Chem. 2009;57:4854–4860. doi: 10.1021/jf900222s. [DOI] [PubMed] [Google Scholar]
- Wang BL, Shi YX, Ma Y, Liu XH, Li YH, Song HB, Liu BJ, Li ZM. Synthesis and biological activity of some novel trifluoromethyl-substituted 1,2,4-triazole and bis(1,2,4-Triazole) Mannich bases containing piperazine rings. J Agric Food Chem. 2010;58:5515–5522. doi: 10.1021/jf100300a. [DOI] [PubMed] [Google Scholar]
- Cudworth DP, Hegde VB, Yap MCH, Guenthenspberger KA, Hamilton CT, Pechacek JT, Johnson PL, Bis SJ, Tisdell FE. Structure-activity relationship development of dihaloaryl triazole compounds as insecticides and acaricides. 1. Phenyl Thiophen-2-yl triazoles. J Agric Food Chem. 2007;55:7517–7526. doi: 10.1021/jf071498s. [DOI] [PubMed] [Google Scholar]
- Chai B, Qian XH, Cao S, Liu H, Song G. Synthesis and insecticidal activityof 1,2,4-triazole derivatives. ARKIVOC. 2003;ii:141–145. [Google Scholar]
- Ma YM, Liu RH, Gong HY, Li Z, Huang QC, Wang HS, Song GG. Synthesis and herbicidal activity of N, N-diethyl-3-(arylselenonyl)-1 H-1,2,4-triazole-1-carboxamide. J Agric Food Chem. 2006;54:7724–7728. doi: 10.1021/jf0609328. [DOI] [PubMed] [Google Scholar]
- Ma Y, Liu R, Gong XY, Li Z, Huang Q, Wang H. Song GG:Synthesis and Herbicidal Activity ofN, N-Diethyl-3-(arylselenonyl)-1 H-1,2,4-triazole-1-carboxamide. J Agric Food Chem. 2006;54:7724–7728. doi: 10.1021/jf0609328. [DOI] [PubMed] [Google Scholar]
- Shi Y, Zhou CH. Synthesis and evaluation of a class of new coumarin triazole derivatives as potential antimicrobial agents. Bioorg Med Chem Lett. 2011;21:956–960. doi: 10.1016/j.bmcl.2010.12.059. [DOI] [PubMed] [Google Scholar]
- Kaplan ZA, Zitouni GT, Ahmet O, Revial G. New triazole and triazolothiadiazine derivatives as possible antimicrobial agents. Eur J Med Chem. 2008;43:155–159. doi: 10.1016/j.ejmech.2007.03.019. [DOI] [PubMed] [Google Scholar]
- Liu JB, Li LC, Dai H, Liu Z, Fang JX. Synthesis and biological activities of new 1 H −1,2,4-triazole derivatives containing ferrocenyl moiety. J Organome Chem. 2006;691:2686–2690. doi: 10.1016/j.jorganchem.2006.02.035. [DOI] [Google Scholar]
- Carter GA, Huppatz JL, Wain RL. Investigations on fungicidesXIX. The fungitoxicity and systemic antifungal activity of certain pyrazole analogues of carboxin. Ann Appl Biol. 1976;84:33. [Google Scholar]
- Kim BJ, Kin J, Kin YK, Choi SY, Choo HYP. Synthesis of benzoxazole amides as novel antifungal agents against malassezia furfur. Bull Korean Chem Soc. 2010;31:1270–1274. doi: 10.5012/bkcs.2010.31.5.1270. [DOI] [Google Scholar]
- Fu J, Cheng K, Zhang ZM, Fang RQ, Zhu HL. Synthesis, structure and structure–activity relationship analysis of caffeic acid amides as potential antimicrobials. Eur J Med Chem. 2010;45:2638–2643. doi: 10.1016/j.ejmech.2010.01.066. [DOI] [PubMed] [Google Scholar]
- WuR ZC, Du XJ, Xiong LX, Yu SJ, Liu XH, Li ZM, Zhao WG. Synthesis, crystal structure and larvicidal activity of novel diamide derivativesagainst Culex pipienspiperine. Chem Cent. 2012;6:99. doi: 10.1186/1752-153X-6-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H, Hu XH, Zou XM, Liu B, Zhu YQ, Wang Y, Hu FZ, Yang HZ. Synthesis and herbicidal activities of novel 3-N-substituted amino-6-methyl-4-(3-trifluoromethylphenyl) pyridazine derivatives. J Agric Food Chem. 2008;56:6567–6572. doi: 10.1021/jf800900h. [DOI] [PubMed] [Google Scholar]
- Wu J, Kang SH, Song BA, Hu D, He M, Jin LH, Song Y. Synthesis and antibacterial activity against ralstonia solanacearum for novel hydrazone derivatives containing a pyridinemoiety. Chem Cent. 2012;6:28. doi: 10.1186/1752-153X-6-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J, Wang J, Hu DY, He M, Jin LH, Song BA. Synthesis and antifungal activity of novelpyrazolecarboxamide derivatives containing ahydrazone moiety. Chem Cent. 2012;6:51. doi: 10.1186/1752-153X-6-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruan YW, Jin LH, Song BA. Synthesis and antifungal activity of new 1-(2,4-dichloro phenyl)-3-aryl-2-(1H-1,2,4-triazol-1-yl) prop-2-en-1-one derivatives. Afr J Pharm Pharmaco. 2011;5:602–607. [Google Scholar]
- Hill GA, Kropa EL. Some halogenated pinacolones. J Am Chem Soc. 1993;55:2509–2512. [Google Scholar]
- Abdul-Ghani MM, Tipping AE. Unsaturated nitrogen compounds containing fluorine - Part 17. The reactions of 3, 5-bistrifluoromethyl)- 1H-1,2,4-triazole with alkynes, alkenes and diazomethane, and of sodium 3,5-bis(trifluoromethyl)-1,2,4- triazolide with halogen compounds. Fluorine Chem. 1995;72:135–145. doi: 10.1016/0022-1139(94)03181-X. [DOI] [Google Scholar]
- Song SQ, Zhou LG, Li D, Tang D, Li JQ, Jiang WB. Antifungal activity of five plants from Xinjiang. Nat Prod Res & Dev. 2004;16:157–159. [Google Scholar]
- Lee JY, Moon SS, Hwang BK. Isolation and in vitro and in vivo activity against Phytophthora capsici and Colletotrichum orbiculare of phenazine-1-carboxylic acid from Pseudomonas aeruginosa strain GC-B26. Pest Manag Sci. 2003;59:872–882. doi: 10.1002/ps.688. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Synthetic route to target compounds 4a to 4v. Synthetic sequence to the novel amide derivatives containing a triazole moiety from intermediate 3.
Yield and elemental analysis data for title compounds 4a to 4v. Description: Structure, yield, and elemental analysis data for title compounds 4a to 4v.
Experimental details and data of the title compounds 4a to 4v. Experimental procedure, spectroscopic data of intermediate 3, title compounds 4a to 4v, copies of 1H NMR, 13C NMR, and IR spectroscopy.


