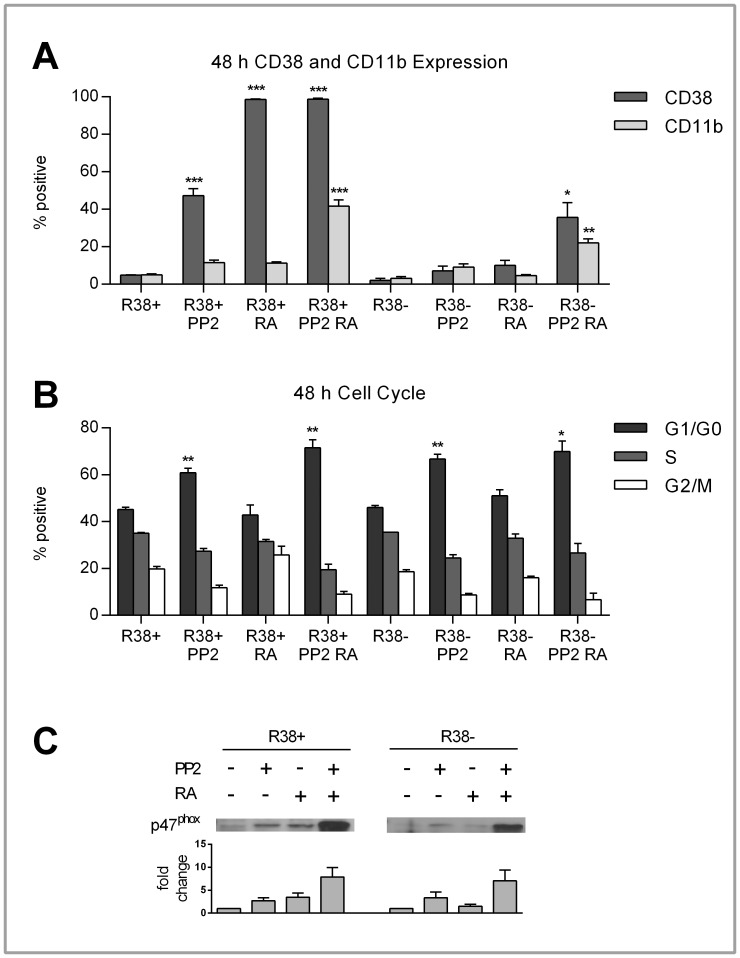
Figure 4. CD38 and CD11b expression, cell cycle progression, and p47phox expression in control, PP2- and/or RA-treated R38+ and R38− HL60 cells.
Error bars represent standard error of at least three repeats. P values were calculated using a student’s t test and are compared between untreated respective control unless otherwise indicated. A: Control, RA-treated and/or PP2-treated R38+ and R38− HL60 cells were labeled with PE-conjugated CD38 and APC-conjugated CD11b and analyzed by flow cytometry. To measure the percent positive signal, controls were set to exclude 95% of the live cell population peak. After 48 h, PP2 induced significant (p<0.001) CD38 expression (∼50%) in R38+. Combined PP2+RA treatment in R38+ significantly increased (p<0.001) CD11b to levels comparable with those of RA-treated WT HL60 cells (∼40%). In R38−, co-treatment resulted in diminished yet still significant increases CD38 (p<0.05, nearly 40%) and CD11b (p<0.01, more than 20%) expression. PP2 treatment alone had no significant effect on CD38 or CD11b expression in R38−. B: To assess cell cycle progression, control and RA-treated WT, R38+ and R38− HL60 cells were stained with propidium iodide-hypotonic solution and analyzed by flow cytometry. Controls were gated to approximately 45% G1/G0 phase, 35% S phase and 20% G2/M phase. PP2 induced growth arrest (more than 60%) in both R38+ (p<0.01 alone and with RA) and R38− (p<0.01 with PP2 alone, p<0.05 with co-treatment). C: p47phox expression was upregulated in both R38+ and R38− with combined PP2+RA treatment. PP2 alone did not increase p47phox expression greater than RA treatment alone in either R38+ or R38−.