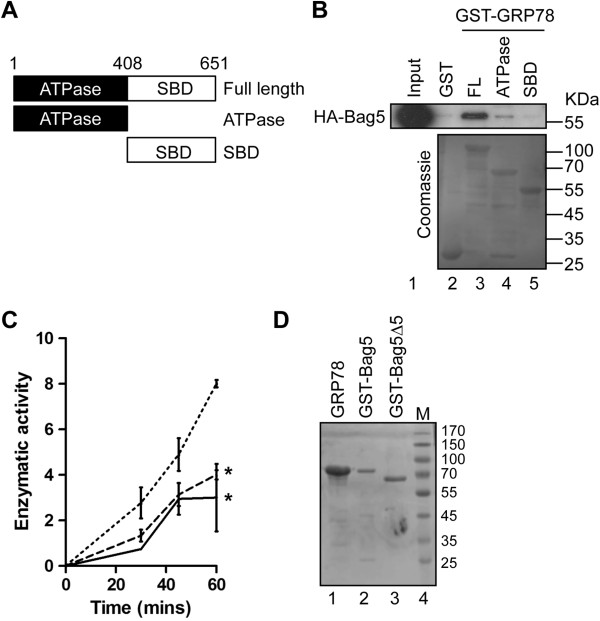
Figure 4.
Bag5 functions as co-chaperone of GRP78/BiP. A. Diagrammatic representation of the molecular chaperone GRP78/BiP and its domains. Numbers indicate the amino acid position. B. Bag5 binds the ATPase domain of GRP78. GST-pull down assay was performed incubating 10 μg of GST-fused protein with 500 μg of HEK-293 cells transfeced with HA-Bag5. A specific anti-HA antibody was used in western blot analysis to detect the binding. Equal recombinant protein employed in the assay was checked by coomassie staining. C. Bag5 enhances GRP78/BiP ATP hydrolysis. ATPase assay with purified GRP78 in absence or presence of Bag5 (dashed line) or Bag5∆ 5 (dotted line). Results are expressed as the average of three independent experiments ± SD (* p < 0.05) D. Coomassie staining of the proteins employed in the ATPase assay.