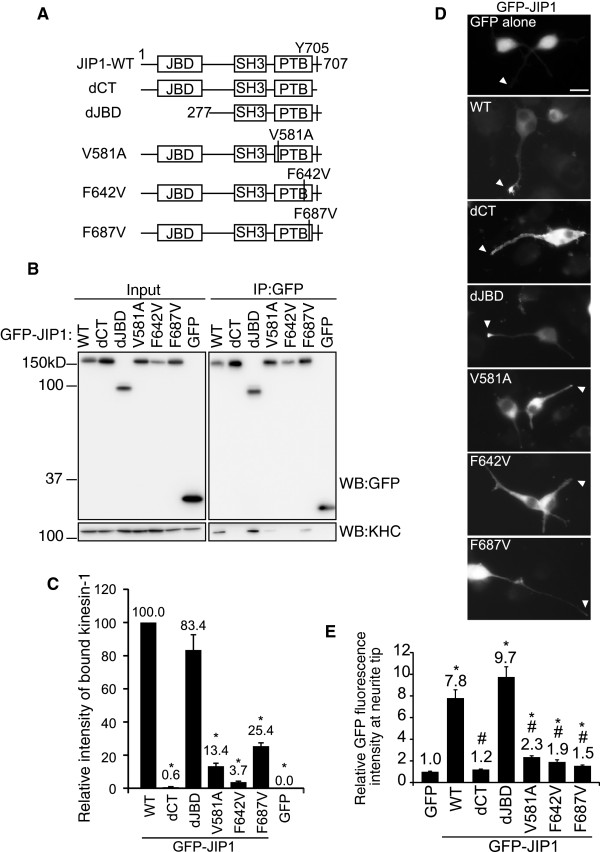
Figure 1.
Formation of the JIP1–kinesin-1 complex in Neuro2a cells is dependent on the JIP1-PTB domain but not JIP1-JBD. (A). Schematic illustration of the JIP1 constructs used in this study. The N-terminal 276 residues were deleted in dJBD. The C-terminal 4 residues were deleted in dCT. V581, F642 and F687 were substituted by A or V in V581A, F642V and F687V. JBD: JNK binding domain; SH3: src homology 3 domain; PTB: phosphotyrosine binding domain; Y705: required tyrosine for kinesin-1 binding. These constructs are GFP- or TAP-tagged at their N-termini. (B). Lysates prepared from Neuro2a cells expressing GFP-JIP1 constructs or GFP alone were immunoprecipitated with anti-GFP antibody and analyzed by western blotting (WB) with the indicated antibodies. Input, cell lysate used for the immunoprecipitation assay. IP:GFP, immunoprecipitated proteins. (C). Quantification of kinesin-1 binding by the GFP-JIP1 constructs in (A). Kinesin-1 binding was normalized to the amount of precipitated GFP-JIP1. *: p < 0.001 compared with GFP-JIP1-WT. Error bars indicate ± SEM. n > 3. (D). Differentiated Neuro2a cells were transfected with expression vectors encoding the GFP-JIP1 constructs shown in (A) or GFP alone. Arrowheads indicate the neurite tips of transfected cells. Scale bar = 20 μm. (E). The neurite tip localization of GFP fusion proteins is shown by the relative fluorescence (tip/shaft ratio) of GFP, compensated by the value of free GFP. *: p < 0.001 compared with GFP. #: p < 0.001 compared with GFP-JIP1-WT. Error bars indicate ± SEM. n = 117 (GFP), 203 (WT), 183 (dCT), 213 (dJBD), 184 (V581A), 64 (F642V), 157 (F687V). These were derived from three independent experiments.