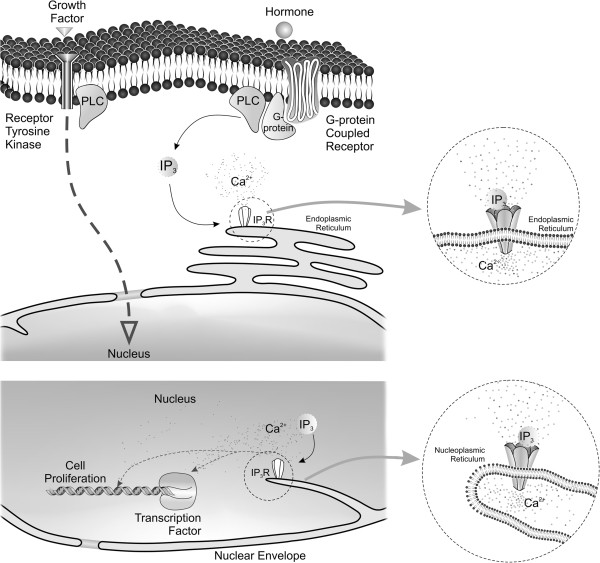
Figure 1.
Schematic representation of events involved in nuclear Ca2+ release and cellular proliferation. Ca2+ signals can be activated in the cells by the binding of a hormone or growth factor to a transmembrane receptor, most commonly a G protein coupled receptor or tyrosine kinase receptor. Through complex signaling cascades and networks, these effectors lead to the activation of several second messengers. One of the signaling pathways activated during cell proliferation is phospholipase C (PLC) that, once activated, cleaves phosphatidylinositol 4,5-biphosphate (PIP2) producing diacylglycerol and inositol-1,4,5-trisphosphate (InsP3). InsP3 then binds to the InsP3 receptor (InsP3R), activating its channel to release Ca2+. InsP3Rs can be found in the endoplasmic reticulum, in the nuclear envelope and in the nucleus along the nucleoplasmic reticulum. InsP3R-induced Ca2+ release specifically in the nucleus has been involved in the regulation of gene expression during different pathophysiological conditions, as well as during cellular proliferation. The cartoon shows the type I nucleoplasmic reticulum structure, with an invagination of the inner nuclear membrane alone. A Type II nucleoplasmic reticulum structure, with a double membrane walled invagination has also been reported in many cell types, although it is not represented here. In the nucleus, these cellular invaginations can provide focal release of Ca2+ that can bind directly to DNA structure or can modulate transcription factors involved in cell proliferation.