Figure 17. Comparison of Dimerization Modes Among Megasynthase Core Domains.
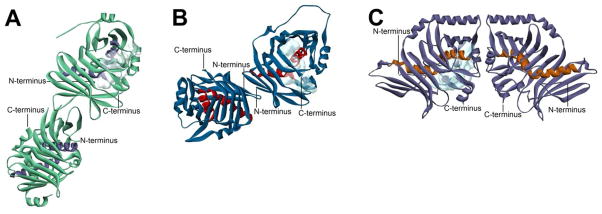
Solid ribbon representations of the core domains from three distinct classes of megasynthase with termini indicated and active site volumes from one monomer shown with a light blue cloud. The images are drawn to scale and superimposable at the monomers with the active site volume shown. (A) L. majuscule CurK DH domain (PDB#3KG9), an example of a modular PKS core, showing a side-by-side dimeric arrangement. (This domain is given the oligomeric state designation of DdhC in Table 1.) (B) S. scrofa FAS DH domain (PDB#2VZ8), an example of a mammalian type I FAS core, showing a tilted side-by-side dimeric arrangement. (This domain is given the oligomeric state designation of DdhD in Table 1.) (C) A. parasiticus PksA PT domain (PDB#3HRQ), an example of a nonreducing IPKS core, showing a unique head-to-head dimeric arrangement. (This domain is given the oligomeric state designation of DdhE in Table 1.)
