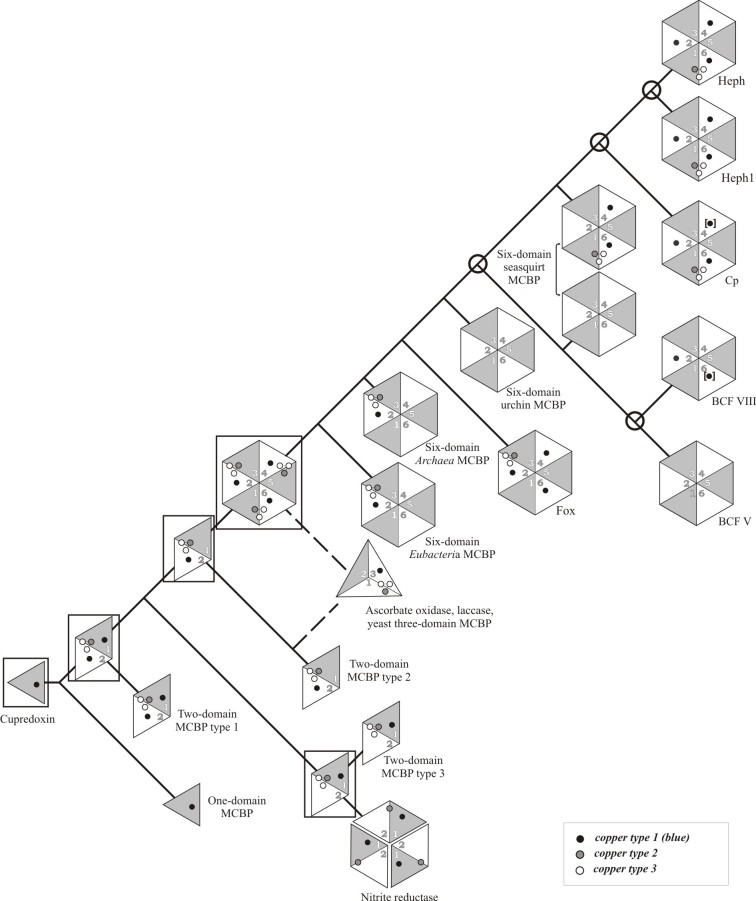
The general scheme of six-domain MCBPs evolution.
The duplication of the parental cupredoxin led to the formation of three types of two-domain proteins with trinuclear copper binding site. The triplication of one of them led to the formation of a hypothetical six-domain protein, from which all modern six- and, possibly, three-domain MCBPs were desended. Hypothetical evolutional ancestors are framed. Two possible evolutional pathways of three-domain proteins formation are marked with dotted lines. Gene duplication events that led to the appearance of five homologous MCBPs in mammals are marked with bubbles. Copper binding sites that could be absent in the proteins of several biological species are indicated in square brackets. MCBP – multicopper blue protein; Cp – ceruloplasmin; BCF – blood coagulation factor; Fox – algae multicopper ferroxidase; Heph – hephaestin; Heph1 – hephestin-like protein 1.